Post-Resuscitation Care
This covers care interventions and supportive care that begin after achieving ROSC. Cardiac arrest, including management, are covered under Cardiac Arrest.
Management
- Prevent further arrest
- Identify cause and consider cardiac catheterisation
- Optimise physiology
- Targeted temperature management to prevent hyperthermia
Resuscitation:
- A
- Secure airway
- C
- Maintain perfusion
Patients usual BP or SBP >100mmHg. - Perform 12-lead ECG
- Maintain perfusion
Specific therapy:
- Pharmacological
- Procedural
- Angiography
- Physical
Supportive care:
- B
- Normoxia
Avoid hypoxia; i.e. SpO2 94-98%. - Normocapnoea
- Normoxia
- D
- Avoid hyperglycaemia
Treat BSL >10mmol/L. May be exacerbated by TTM. - Treat seizures
- Remain sedated
- Avoid hyperglycaemia
- E
- Targeted Temperature Management
Keep temperature 35.5-36.5°C for at least 24 hours, and maintain normothermia (<37.5°C) for at least 72 hours.- Active external cooling using a feedback-controlled system, guided by bladder or oesophageal temperature
- Should be achieved within 8 hours of ROSC
- Control of shivering:
- Tier 1
- Paracetamol 1g Q6H
- Mg2+ >0.8mmol/L
- Tier 2
- Wrap hands and feet with warm towels
- Dexmedetomidine or clonidine
- Remifentanil
- Ketamine
- Mg2+ 1.2-1.6mmol/L
- Tier 3
- Fentanyl boluses
- Paralysis
May be needed to control shivering. Consideration for EEG monitoring if paralysing seizing patients.
- Tier 1
- Targeted Temperature Management
A variety of cooling methods have been tried, although with change in aims from hypothermia to normothermia many of the more aggressive methods are no longer relevant. These are covered in detail under Fever and Hyperthermia.
Disposition:
- Catheter laboratory
- ICU
Preventative:
Immediate:
Key Studies
Many key trials target only OOHCA or IHCA patients, even though therapy could conceivably benefit either group.
It is unclear how generalisable results in one group are to the other, given the disparities in aetiology and outcome.
This includes key trials evaluating interventions that occur after achieving ROSC, such as angiography and hypothermia. Trials evaluating the conduct of ALS are covered under Cardiac Arrest.
Angiography following OOHCA:
- PCI has clear mortality benefits in STEMI, but not in NSTEMI
- CAD is highly prevalent in patients with OOHCA
Culprit (>50% stenosis) coronary lesions are found in ~1/3rd of OOHCA without STE. - COACT (2019)
- 538 Dutch patients following OOHCA with an initial shockable rhythm and unconsciousness following ROSC
- Randomised to immediate angiography vs. delayed angiography
- Immediate angiography
- 33% PCI, 6% CABG
- Delayed angiography
- Occurred following neurological recovery or new indication developed
- 24% PCI, 8% CABG
- Angiography for any patient with indications
Ischaemia, cardiogenic shock, arrhythmias. - All unstable coronary lesions treated
- Immediate angiography
- No 90 day mortality difference (64.5% vs 67.2%, CI 0.62-1.72)
- No difference in secondary outcomes of survival with good neurological outcome, survival to ICU DC, or organ supports
- Low coronary intervention rate
- TOMAHAWK (2021)
- 554 German and Danish patients aged >30 following OOHCA
- Without obvious cardiac cause (STE or LBBB), or obvious non-cardiac cause (TBI)
- Investigator-initiated, open label, block-randomised RCT
- Immediate vs. delayed angiography
- Immediate angiography
- Delayed angiography
- Angiogram >24 hours if high likelihood of coronary cause
- Immediate angiography if obvious cardiac cause
- All clinical relevant lesions had revascularisation attempted
- No change in 30 day mortality (54% vs 46%, CI -0.6-16.4%)
- Powered for 12% ARR mortality difference, seems ambitious
- 17% of delayed group had angiogram <24 hours
Hypothermia:
- Patients with cardiac arrest due to hypothermia have good outcomes despite prolonged resuscitation
- Hypothermia has several potentially beneficial effects:
- Anticonvulsant
- Anti-inflammatory
- ↓ Cerebral oedema and ICP
- ↓ CMRO2 and ↓ HIE
- Hypothermia also has several potential harms
- ↓ HR and ↓ CO
- Arrhythmias
- ↑ QTc
- ↓ Immune function
- ↓ Rate of drug metabolism
- Hyperglycaemia
- HACA (2002)
- 275 non-pregnant European adults with witnessed shockable cardiac arrest of presumed cardiac origin
- Multicentre, assessor-blinded, block-randomised RCT
- Favourable outcome defined as CPC of 1-2; no clear power calculation
- Hypothermia vs. standard care
- Hypothermia group
- 32-34°C with surface cooling for 24 hours
- Passive re-warming after 24 hours
- Standard care group
- Hypothermia group
- Significant ↑ in favourable neurological outcome (55% vs. 39%) and ↓ in mortality (41% vs. 55%) in hypothermic group
- Normothermia group became hyperthermic after ~ 12 hours
- Stopped early for slow recruitment
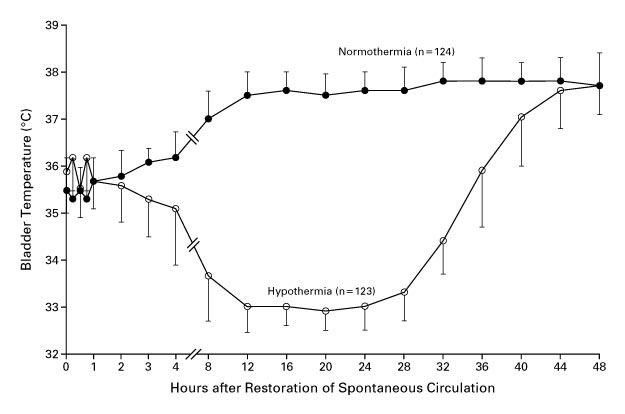
- TTM (2013)
- 950 adults with OOHCA from presumed cardiac cause and GCS <8
- Multicentre (36), assessor blinded RCT
- 900 patients provide 90% power to detect 20% ↓ in hazard ratio for death
This is ~11% ARR, which is very ambitious. - Therapeutic hypothermia at 36°C vs. 33°C
Both groups:- Targeted management for 28 hours
- Invasive or surface cooling
- Gradual warming to 37°C by 0.5°C/hr
- Avoidance of fever for 72 hours
- No mortality difference (50% vs. 48%; hazard ratio 0.89 (CI 0.89 to 1.28))
But probably underpowered for this outcome. - Reasonably good separation between groups
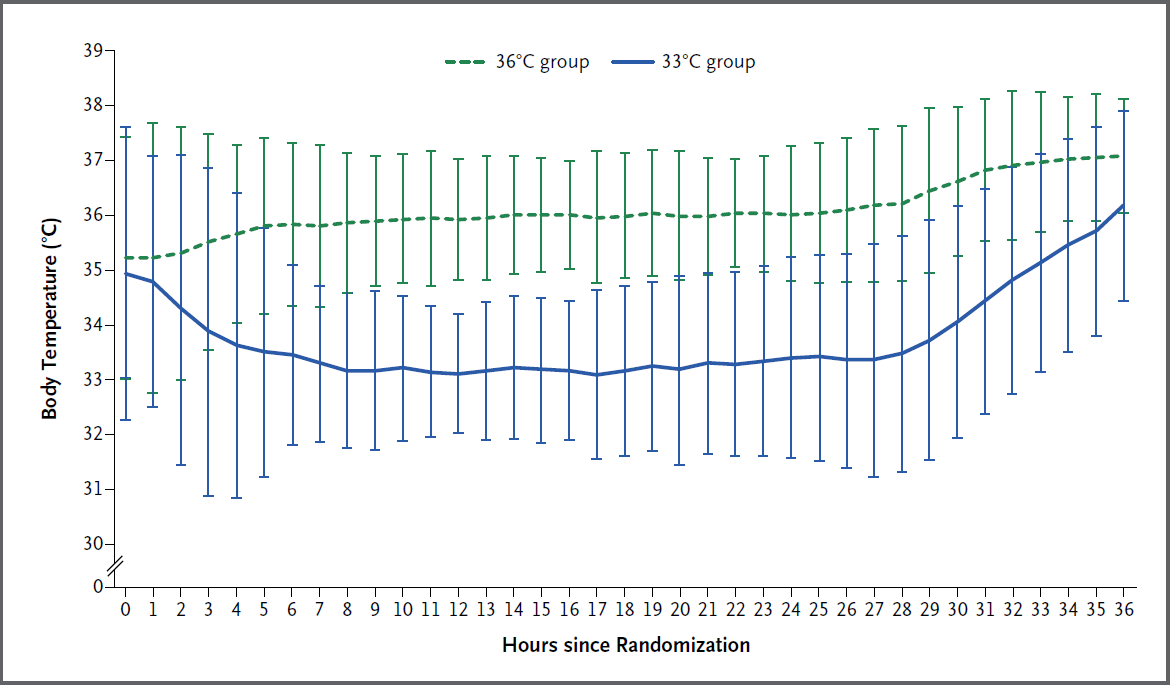
- HYPERION (2019)
- 584 non-pregnant, non-lactating Frenchpersons without end-stage liver disease post IHCA or OOHCA with a non-shockable rhythm of any cause with GCS <8 prior to admission, with <10 minute no-flow time and <60 minute low-flow time, not on obscene doses of vasopressors (<1ug/kg/min of nor/adrenaline)
- Randomised, single assessor, assessor-blinded, multi-centre RCT
- 80% power for 9% ↑ in survival with favourable neurological outcome, assuming 14% in control group
- Hypothermia (33°C) vs normothermia (36.5-37.5°C)
- Hypothermia
- Maintained for 24 hours
- External or internal active devices used
- Rewarmed at 0.25-0.5°C/hr
- Sedation to RASS -5
- Normothermia
- Maintained for 48 hours
- External or internal active devices used
- Sedation to RASS 0
- Routine sedation only for first 12 hours
- Hypothermia
- ↑ Survival with favourable outcome in hypothermia group (10.2% vs. 5.7%)
Fragile result, particularly with subjective outcome. - 30% had no bystander CPR
- 4-9% were unwitnessed arrests (lower in hypothermia group)
- 5% of control group had fevers
- Greater overlap of temperatures compared to TTM
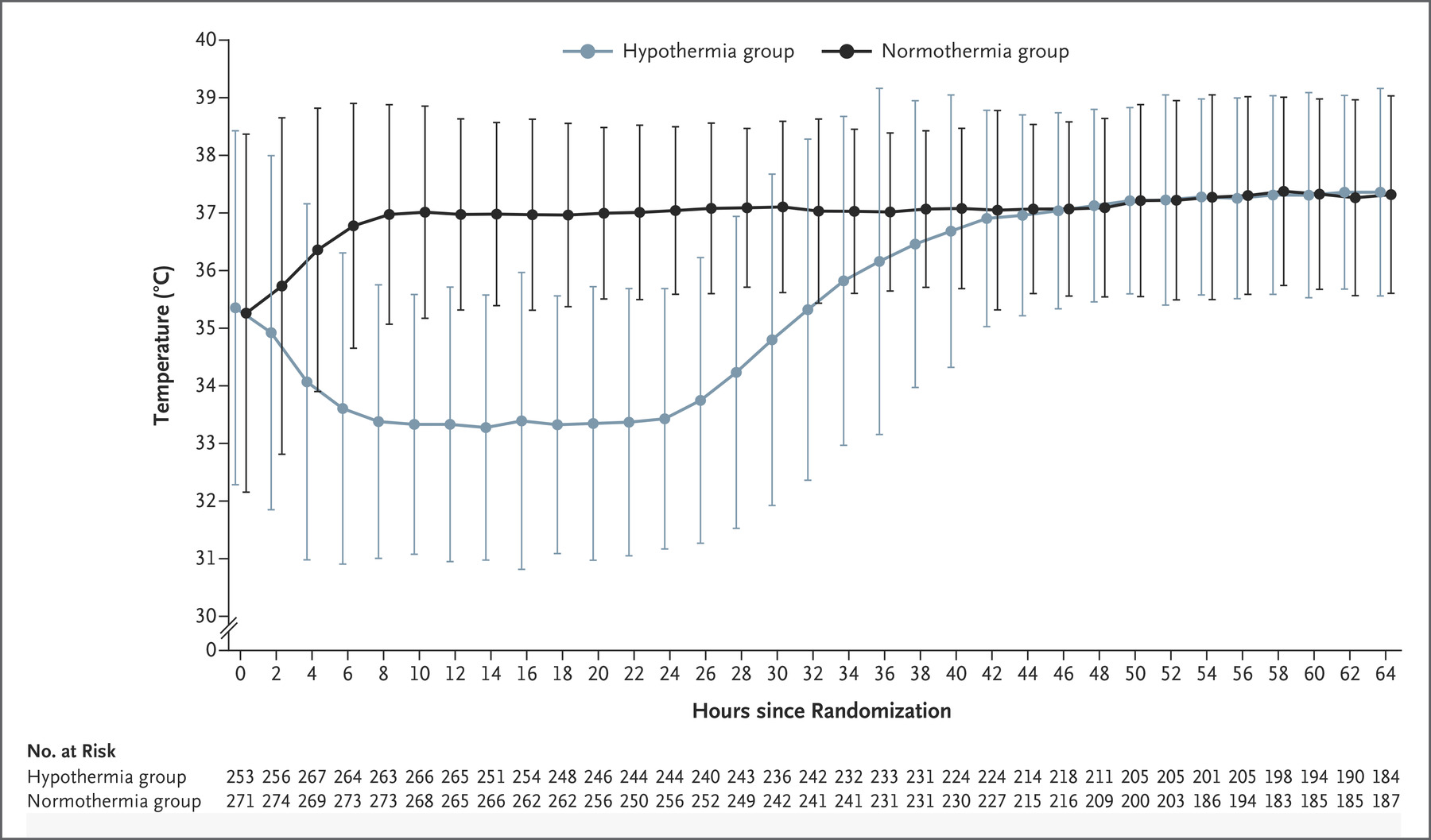
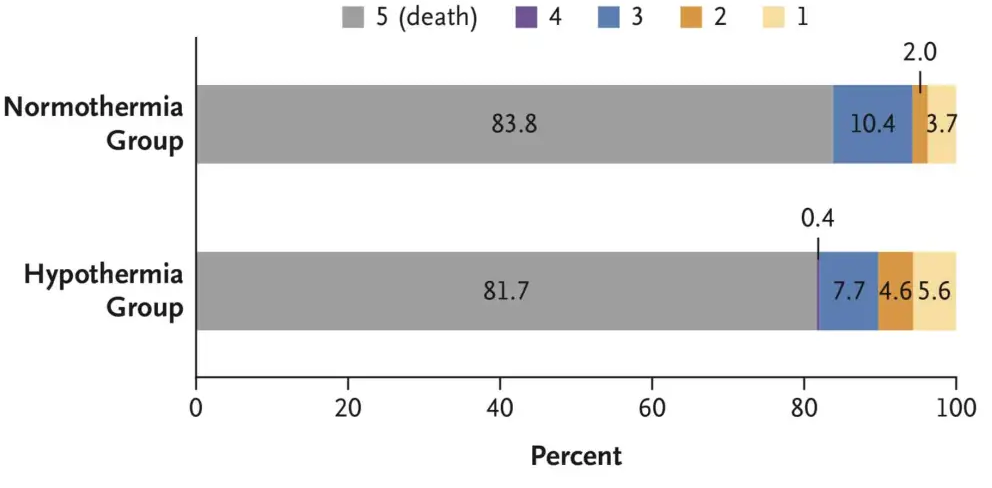
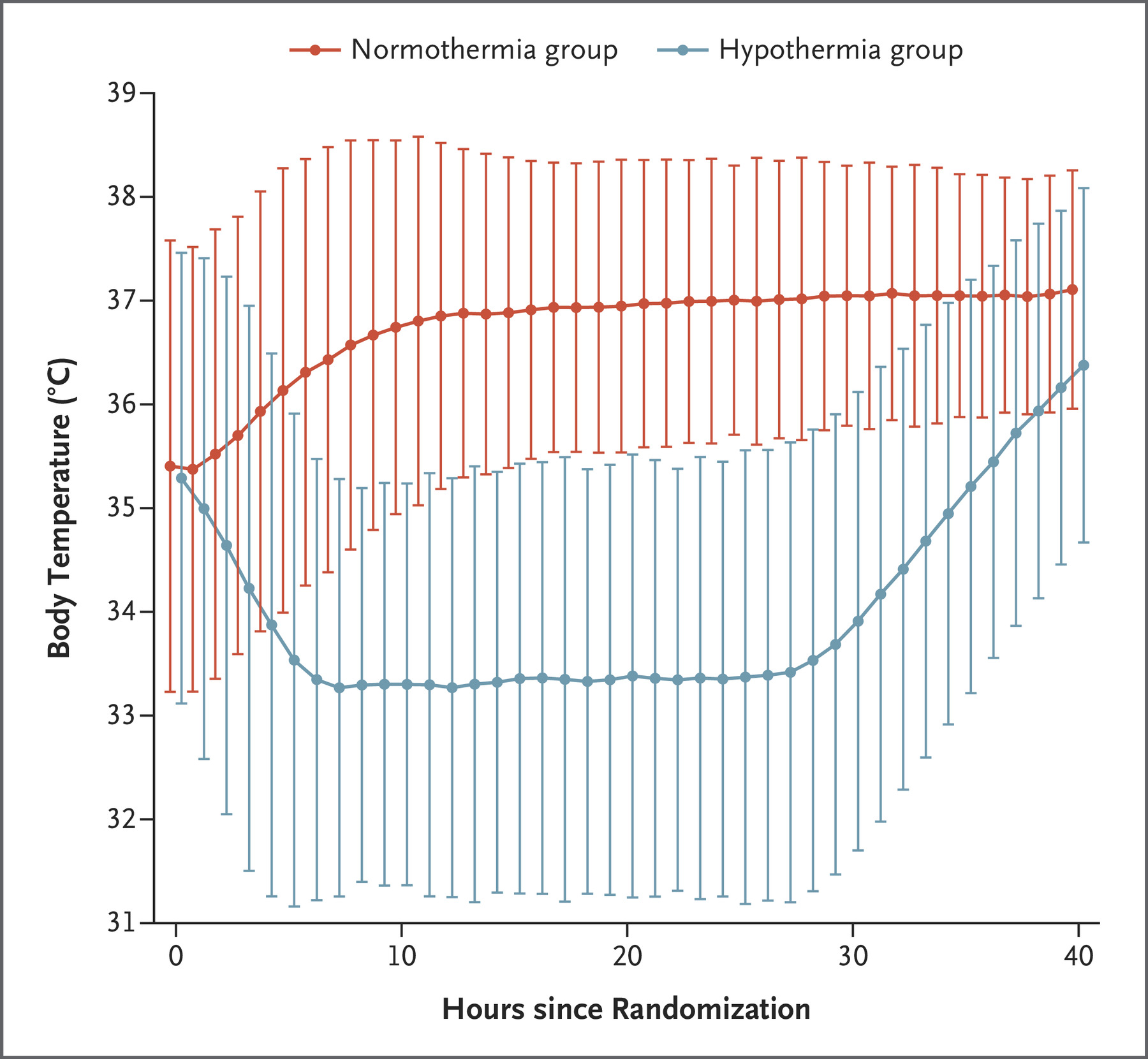
- TTM2 (2021)
- 1861 non-pregnant patients across 14 countries with witnessed OOHCA with sustained ROSC and FOUR score <4 without: ECMO, treatment limitations, ICH
- International, multi-centre, parallel group RCT
- Randomised to hypothermia (33°C) vs. normothermia (36°C)
- Hypothermia group
- Rapidly cooled with cold IVT and active internal or external cooling
- Rewarming commenced after 28 hours at <0.3°C/hr
- Normothermia group
- Cooled to ⩽37.5°C
- Less-invasive therapies initiated first
- Both groups had temperature managed using a feedback-controlled system
- Hypothermia group
- Median temperature in hypothermia group at 3 hours was 34°C, reflecting degree of difficulty achieving hypothermia
- No difference in 180 day mortality (50% vs 48%)
- ↑ Arrhythmias in hypothermia group (24% vs. 16%)
- Definitive trial on this question
Other:
- BOX (2022)
- Theorised that hyperoxia may ↑ neuronal and lung injury due to ↑ reactive oxygen species, and lead to coronary and cerebral vasoconstriction which may worsen HIE
- 802 patients in admitted in 2 Danish ICUs following OOHCA with ROSC after 20 minutes and GCS <8
- Oxygen arm:
- Restrictive oxygenation (PaO2 68-75mmHg) vs. liberal oxygenation (98-105mmHg)
Randomisation occurred in hospital, usually in ICU. - No change in mortality or neurological outcome
- Unblinded
- Restrictive oxygenation (PaO2 68-75mmHg) vs. liberal oxygenation (98-105mmHg)
- MAP arm:
- MAP 77mmHg vs MAP 63mmHg
- Protocolised therapy with fluids, noradrenaline, and dopamine
- No change in mortality or neurological outcome
- Blinded
- All patients received 24 hours of TTM
- TAME (2023)
- Theorised that mild hypercapnoea may lead to cerebral vasodilatation, ↑ CBF, and ↑ neurological outcome
- 1700 non-pregnant, non-head injured adults with witnessed OOHVFA of presumed cardiac or idiopathic cause with >20 minutes of ROSC
- Multicentre (63), international, assessor-blinded, allocation concealed, RCT
- Mild hypercapnoea (50-55mmHg) vs. normocapnoea for 24 hours
- Severe metabolic acidosis corrected prior
- Normocapnoea restored over 24-48 hours in hypercapnoic group
- RASS -4 in both groups
- No change in favourable outcome (43.5% vs. 44.6%)
- Good separation of groups
The Glasgow Outcome Scale - Extended (GOS-E) is commonly used to measure TBI outcome:
- Death
- Vegetative state
Unaware, periods of spontaneous eye opening, persistent reflex responses. - Lower severe disability
Dependent, unable to be left alone for >8 hours. - Upper severe disability
Dependent, able to be left alone for >8 hours. - Lower moderate disability
Independent at home, dependent outside, unable to return to work. - Upper moderate disability
Independent at home, dependent outside, able to return to work with special arrangement. - Lower good recovery
Some disability but able to return to work. - Upper good recovery
Some deficits but not disabling.
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Schmidt H, Kjaergaard J, Hassager C, et al. Oxygen Targets in Comatose Survivors of Cardiac Arrest. New England Journal of Medicine. 2022;387(16):1467-1476. doi:10.1056/NEJMoa2208686
- Andersen LW, Isbye D, Kjærgaard J, et al. Effect of Vasopressin and Methylprednisolone vs Placebo on Return of Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326(16):1586–1594. doi:10.1001/jama.2021.16628