Sepsis
A life-threatening organ dysfunction due to a dysregulated host response to infection, where:
The definition of sepsis has remained necessarily vague, as the clinical diagnosis of sepsis is based on a constellation of non-specific features. Drawing a line which neatly includes the septic and excludes other illnesses is therefore not currently possible.
This is the Sepsis-3 definition, which moved the definitional goalposts from trying to pin down “sepsis” to trying to identify patients with clinically suspected infection who had a high mortality.
- Organ dysfunction
⩾2 ↑ in SOFA score. - Septic shock
Sepsis, with:- ↓ BP requiring vasopressors
- Lactate >2mmol/L
Management of febrile neutropaenia and neutropaenic sepsis is covered under Febrile Neutropaenia.
Epidemiology and Risk Factors
Major global healthcare burden:
- High number of cases
- High mortality
~30%.
Host response is highly heterogenous, and is affected by:
- Patient factors:
- Comorbidities
- Genetics
- Pathogen factors:
- Pathogen virulence
- Location of infection
| Immune Deficiency | Causes | Bacteria | Fungi | Viruses |
|---|---|---|---|---|
| Neutrophils |
|
|
|
|
| Monocytes/Macrophages |
|
|
|
|
| B lymphocytes |
|
|
||
| Humoral |
|
|
||
| T lymphocytes |
|
|
|
|
Pathophysiology
Progression from a localised infection to systemic involvement requires:
- Activation of pattern-recognition receptors
- Toll-like receptors
- C-type lectin receptors
- Retinoic acid inducible gene-1-like receptors
- Receptor activation leads to complex, simultaneous alteration of multiple metabolic pathways:
- Pro-inflammatory
- Anti-inflammatory
Simultaneously with pro-inflammatory processes. - Neurohormonal
- Metabolic change
- Coagulation activation
Tend towards a prothrombotic, antifibrionolytic state which may promote microvascular thrombosis and organ ischaemia. - Macrovascular dysfunction
- Microvascular dysfunction
- Endothelial dysfunction
- Glycocalyx disruption resulting in ↑ fluid extravasation
Immune paralysis:
- Survival of the initial septic phase may lead to:
- A dysfunctional immune system
- Infections classically associated with immunocompromise
Vasodilation occurs via several mechanisms:
- Acidosis
- K+ efflux leading to membrane hyperpolarisation
↓ Ca2+ entry into vascular smooth muscle. - Catecholamine resistance
- K+ efflux leading to membrane hyperpolarisation
- ↑ NO production
Induced by cytokines and bacterial endotoxin. - Adrenal suppression
- Endogenous vasopressin suppression
Septic cardiomyopathy:
- ↓ Diastolic function
Aetiology
Any progression of localised infection may lead to sepsis; the most common in adults include:
- Lung
~60%. - Abdominal
~20%. - Primary bacteraemia
- Renal or GU
Lung causes are less common in children, with the difference made up with CNS infections and ↑ primary bacteraemia.
| Organ System | Common | Uncommon | ICU-Associated |
|---|---|---|---|
| Systemic |
|
|
|
| Respiratory |
|
|
|
| Cardiovascular |
|
|
|
| Neurological |
|
|
|
| Genitourinary |
|
|
|
| Gastrointestinal |
|
|
|
| Integumentary |
|
|
|
Notes:
|
|||
| Source | Gram Positive Cocci | Gram Negative Bacilli | Other |
|---|---|---|---|
| CAP/HCAP |
|
|
|
| HAP/VAP |
|
|
|
| Primary bloodstream |
|
|
|
| Endocarditis |
|
|
|
| Meningitis and Encephalitis |
|
|
|
| Healthcare-associated CNS infection |
|
|
|
| Skin and Soft Tissue |
|
|
|
| Intra-abdominal |
|
|
|
| Urinary Tract |
|
|
|
| Surgical Site |
|
|
|
| Intravascular device |
|
|
|
| Multisystem |
|
|
|
| NB: Pathogens in bold have increased risk of multi-drug resistance. | |||
Clinical Features
Heterogenous and non-specific features:
- Tachycardia
- Temperature
- 35% normothermic
- 10% hypothermic
- 55% hyperthermic
- Often fluctuant
- Less likely to be present in the:
- Elderly
- Immunosuppressed
- Malnourished
Assessment
History
| Factor | Details | Causative Organisms |
|---|---|---|
| Healthcare | Recent hospital admission |
|
| Antibiotic |
|
|
| Lines and devices |
|
|
| Blood Transfusion |
|
|
| Leech use |
|
|
| Travel history | Tropical |
|
| LMIC |
|
|
| Hospitalised |
|
|
| Lifestyle | Alcohol |
|
| IVDU |
|
|
| Domestic | Infectious contact |
|
| Spa |
|
|
| Air conditioning |
|
|
Institutionalised:
|
|
|
| Animals | Domestic companions:
|
|
| Birds |
|
|
| Rodents |
|
|
Farm animals:
|
|
|
| Horses |
|
|
| Ticks |
|
|
| Bats |
|
|
| Occupational | Healthcare |
|
| Soil contact |
|
|
| Treated water |
|
|
| Immunocompromise | Vaccination |
|
| Pregnant |
|
|
| Splenectomy |
|
|
| Immunosuppressed |
|
|
| Food | Unpasteurised milk |
|
| Undercooked meat and eggs |
|
|
| Shellfish |
|
Exam
Investigations
Laboratory:
- Blood
- CRP
- Non-specific marker of inflammation
- May be more specific for Strep. pneumoniae infection
This may also better identify patients in whom steroid would be appropriate.
- May be more specific for Strep. pneumoniae infection
- Rises 4-6 hours after onset of infection, doubles ~8 hourly
- Correlates with severity of infection
- Rapid ↓ indicates response
- Non-specific marker of inflammation
- Procalcitonin
- Relatively more specific marker of inflammation
Elevated in:- Bacterial infection
Produced in response to bacterial endotoxin.- Levels rise in 6-8 hours
Prior to cultures flagging positive. - Not ↑ in viral or fungal infections
- Not ↑ in local bacterial infection without a systemic response
- Levels rise in 6-8 hours
- Burns
- TLS
- Major surgery
- Multi-organ failure
- ESRD
Renally cleared.
- Bacterial infection
- No better than clinical judgment in discriminating infectious vs. non-infectious causes
- Expensive, requires serial measurements
- Relatively more specific marker of inflammation
- Blood cultures
Prior to antibiotics. - Galactomannan
- Presence suggestive of fungal infection, particularly aspergillosis
- Risk of false positives from other fungal infections or concomitant β-lactam use
- (1→3)-β-D-glucan antigen
Cell wall component of most fungi. Improves sensitivity and specificity in combination with galactomannan.
- CRP
Diagnostic Approach and DDx
Diagnosis of sepsis is difficult.
- Clinical
- Signs are non-specific
- Changes with local infection
- May not be present in:
- Elderly
- Immunocompromised
- Findings may reflect other forms of shock
- Signs are non-specific
- Laboratory
- Markers are non-specific
- Microbiological samples:
- Take significant time to process
Requires treatment to be initiated in advance of knowing. - Must distinguish infection from colonisation
Requires clinical interpretation.
- Take significant time to process
The qSOFA is a quick screening tool to identify patients who should be considered for sepsis workup, and requires ⩾2 of:
- Altered mentation
- RR >22
- SBP <100mmHg
There is a substantial overlap in features with HLH (see Haemophagocytic Lymphohistiocytosis); consider HLH as a differential diagnosis in septic patients without a source.
Management
- Early antibiotics (<1 hour) with blood cultures (2-3 sets) prior
- Determine haemodynamic goals and target with:
- Fluids
- Vasopressors
Resuscitation:
Each hour delay in antibiotic administration is associated with a 12% ↓ in survival.
- C
- Target MAP >65mmHg
Consider ↑ (e.g. MAP >70mmHg) if renal failure, poorly controlled hypertension. - Fluid resuscitation
10-20mL/kg up to 30mL/kg total.- Crystalloid most effective
- Albumin equivalent outcomes with potentially ↓ total volume delivered, and haemodynamic goals are achieved move quickly
- Starch harmful
- Use dynamic measures of fluid responsiveness to assess need for more therapy
- Passive leg raise
- Pulse pressure variation
- Crystalloid most effective
- Arterial line
If vasopressors required. - Vasopressors
- Noradrenaline 1st line
- ↑ Preload due to venoconstriction
- ↑ SVR due to vasoconstriction
- Maintain or ↑ CO
B1 effects ↑ CO, compensating for ↑ in afterload.
- Consider adrenaline as 2nd line
- Vasopressin as 3rd line
- End-of-the line vasopressor options without much supporting evidence include:
- Methylene blue
1mg/kg bolus over ~30 minutes. - Hydroxycobalamin
5g. - Terlipressin
- Angiotensin II
- Methylene blue
- Noradrenaline 1st line
- Assess CO
- Inotropic support if:
- Adequately volume resuscitated
- Evidence of ↓ perfusion:
- ↑ Lactate
- ↓ Central capillary refill
- Echocardiography
- Pre-existing LV dysfunction
- VA ECMO
Appropriate in selected patients with myocardial dysfunction, acknowledging high mortality of this cohort.
- Inotropic support if:
- Target MAP >65mmHg
Early use of noradrenaline is associated with ↓ mortality.
Average total IV fluid resuscitation at:
- 6 hours is ~4.2L ±1.4L
- 72 hours is ~6.8L ±3L
Specific therapy:
- Pharmacological
- Antibiotics
- Empirical
Tailored to likely sources and resistance patterns.
- Empirical
- Corticosteroids
- Remain somewhat controversial
- Appropriate for patients:
- With another indication for steroids
- Sepsis with CAP
- May be appropriate in septic patients:
- Refractory to vasopressors
- Otherwise high risk
- Unlikely to change mortality or outcome
- Will spare vasopressors
- May ↓ ventilator days and speed shock resolution
- Toxic Shock cover
If treating TSS, or empirical if clinically likely.- Clindamycin
- IVIG
- Antibiotics
- Procedural
- Source control
- Removal of short-term intravascular catheters if catheter-related sepsis is likely
This includes arterial catheters.
- Physical
My approach is to use corticosteroids in septic patients with:
- CAP
- On previous steroid supplementation
- With noradrenaline ⩾0.15μg/kg/min
Supportive care:
- F
- pH >7.15
↑ Cardiac sensitivity to catecholamines.- Sodium bicarbonate
- THAM
- iCa >1.1mmol/L
Very low supporting evidence.
- pH >7.15
- H
- Hb >70
Disposition:
Preventative:
Marginal and Ineffective Therapies
Drugs:
- β-blockade
- Esmolol probably most well studied
- Very high control group mortality
- May reflect reversal of harmful exogenous β-agonists
- Esmolol probably most well studied
- Activated protein C
- Vitamin C
- IVIG
No proven role outside of TSS.
Resuscitation targets:
- CVP targeting
- ScvO2 targeting
- PAC targeting
Use of PAC does not improve and may worsen outcome.
Blood Purification:
- High-volume haemofiltration
CRRT with target dose >35mL/kg/hr. - Polymyxin B Haemoperfusion
CRRT with Polymyxin B bound to the filter, which binds bacterial endotoxin. - High cut-off haemofiltration
CRRT with larger pores, allowing filtration of middle-molecular weight proteins which include pro- and anti-inflammatory cytokines.
Other:
- Hydroxy-ethyl starch resuscitation fluids
↑ AKI and mortality.
Anaesthetic Considerations
Complications
Complications of sepsis include:
- B
- ARDS
- C
- Septic cardiomyopathy
- D
- Septic encephalopathy
Impaired mental function in the setting of extracranial infection.- 10-80% of septic cases
- ↑ Mortality associated with ↓ GCS
- Potential contributors include:
- Bacterial endotoxin
- Cytokine release
- Haemodynamic collapse
- Septic encephalopathy
- F
- AKI
Septic AKI
Epidemiology of septic AKI:
- Occurs in 22% of ICU patients with sepsis
- Associated with ↑ in mortality to 38%
Management:
- Preventative
Most effective method:- Low dose vasopressors
Achieving a MAP >80mmHg may reduce requirement for RRT. - Note that excessive IV fluid is ineffective
- May worsen oedema
Aim euvolaemia. - Septic AKI is not a low-flow state
- Balanced solutions are beneficial
- Avoid starch and gelatin colloids
- May worsen oedema
- Treat the sepsis
- Low dose vasopressors
Prognosis
High mortality:
- ICU death 28-40%
~30% when adjusted for severity.
Key Studies
Fluid:
- FEAST (2011)
- 3170 African children aged 60 days to 12 years with febrile illness, ↓ conscious state, and ↓ perfusion; without malnourishment, gastroenteritis, or non-infectious shock
- Multicentre (6), un-blinded, allocation concealed, block-randomised trial
- 3600 patients would provide 80% power for 5% ↓ ARR of death, assuming control mortality of 11%
- Patients without severe hypotension (3141) randomised to one of:
- 20-40mL/kg 0.9% saline
- 20-40mL/kg 5% albumin
- No fluid
- Volumes of fluid were ↑ (from 20 to 40mL) after a protocol amendment partway through the trial
- Fluid groups received additional fluid if impaired perfusion
- All patients treated on general paediatric wards (no ICU available), and transfused if Hb <5g/dL
- 48 hour mortality was significantly ↑ in fluid groups
Saline 10.5%, albumin 10.6%, control 7.3%.- RR for saline vs. control: 1.44 (CI 1.09-1.9)
- Stopped early for harm
- Most deaths occurred at <24 hours
- Weaknesses:
- Clinical criteria for shock diagnosis are non-specific
- >50% had malaria, which behaves differently with IVT
- Significant anaemia may be made worse by haemodilution
A separate arm of FEAST protocolised management of severe hypotension, but there were only 29 patients and the discussion adds complexity disproportionate to insight so I have excluded it from this summary.
- ARISE (2014)
- 1600 non-pregnant adults with sepsis and refractory hypotension or hypoperfusion who had commenced antibiotics
- Multi-centre (51), unblocked, block-randomised trial
- EGDT vs. control
- EGDT
- River’s algorithm used
- Control
- Usual care
- Arterial line and CVC permitted
- ScVO2 measurement not permitted
- EGDT
- No difference in all cause mortality (18.6% vs. 18.8%)
- SAFE (2004)
- ~7000 Australian adult ICU patients requiring fluid administration, excluding post-cardiac surgery, liver transplant, and burns
- Multicentre, double-blinded RCT, stratified by trauma diagnosis and site
Special fluid administration sets were used to hide the albumin. - 4% albumin vs. 0.9% saline
- No change in 28-day mortality (20.9% vs. 21.1%)
- No statistically significant difference in ICU length of stay, duration of organ support. However:
- Trauma subgroup had an almost significant ↑ mortality (13.6% vs. 10%, RR 1.36 (CI 0.99-1.86))
- Sepsis subgroup had a more insignificant ↓ mortality (30.7% vs. 35.3%, RR 0.87 (CI 0.74-1.02))
- Trauma subgroup had an almost significant ↑ mortality (13.6% vs. 10%, RR 1.36 (CI 0.99-1.86))
- Less cumulative fluid balance at end of day 1 and 2 in the albumin group (by ~1:1.4), although this difference started resolving at day 3 and had gone at day 4
Note that 4% Albumin is hypotonic at 260mOsmol/L. This may contribute to the poor outcomes seen in the neurosurgical group, and is explored elegantly by Iguchi et al.
- ALBIOS (2014)
- 1818 Italians within 24 hours of severe sepsis, without head injury, heart failure, or specific indication for albumin
- Multicentre (100) open-label, randomised trial, with stratification by ICU and time of sepsis onset
- 80% power for 7.5% ARR (!) ↓ in 28-day mortality, with control mortality of 45%
- 20% albumin vs. crystalloid
- 20% albumin
- 300mL 20% albumin on randomisation
- Further 20% targeting serum albumin >30g/L
- Crystalloid as clinically indicated
- Crystalloid
- Crystalloid as clinically indicated
- 20% albumin
- No mortality difference (31.8% vs. 32%)
- 20% albumin is safe and improves haemodynamics, but does not provide a survival advantage
- CLOVERS (2023)
- ~12,000 patients with sepsis and hypotension after 1L IVT
- Within 24 hours of hospital admission
- <3L of IVT
i.e. 1-3L of IVT received by randomisation.
- Restrictive vs. liberal fluid strategy
- Restrictive: Up to 2L IVT, then noradrenaline
Rescue fluids allowed. - Liberal: 2L bolus at randomisation, further 500mL boluses
Rescue vasopressors allowed, and recommended after 5L (6-8L total) IVT.
- Restrictive: Up to 2L IVT, then noradrenaline
- No change in mortality, ventilator free days, ICU free days, ARDS
- ↑ ICU admission in restrictive group
- Strong safety profile of peripheral vasopressors
- ~12,000 patients with sepsis and hypotension after 1L IVT
- CLASSIC (2022)
- ~1500 non-pregnant, non-burned Europeans with sepsis within 12 hours of diagnosis
- Restrictive vs. liberal fluid strategy
- Restrictive: Crystalloid given for severe hypoperfusion, overt losses, or to ensure at least 1L/24 hours
- Liberal: Crystalloid given based on surviving sepsis guidelines, to replace losses
- Vasopressors as per trial protocol
- No difference in mortality
- Small difference in volume administered (~2L at day 5)
Early Goal Directed Therapy:
- Early Goal-Directed Therapy is (?was) a protocolised pathway for sepsis management, targeting specific haemodynamic goals in order to maximise DO2 and thus restore cellular oxygen balance:
- CVP 8-12mmHg
- MAP 65-90mmHg
- UO >0.5mL/kg/hr
- ScvO2 >70%
- Haematocrit >30%
- The comfort of this physiological approach did not survive the crucible of real-word RCT evaluation, and EGDT has been subsequently dismissed
- The principles of aggressive, goal-directed (but not CVP and ScvO2-directed) therapy live on
- Rivers et al (2001)
- Single-centre, non-randomised, non-blinded trial
- High control group mortality (46.5%)
- Significant hospital ↓ mortality in EGDT arm (30% vs. 46%)
- Use of ScvO2 target was not based on prior evidence
- Effect may be due to presence of experience clinician at the bedside directing therapy
- ARISE (2014)
- 1600 non-pregnant adults with sepsis and refractory hypotension or hypoperfusion who had commenced antibiotics
- Multi-centre (51), unblocked, block-randomised trial
- EGDT vs. control
- EGDT
- River’s algorithm used
- Control
- Usual care
- Arterial line and CVC permitted
- ScVO2 measurement not permitted
- EGDT
- No difference in all cause mortality (18.6% vs. 18.8%)
- ProCESS (2014)
- 1,351 American adults with sepsis admitted from the emergency department
- Multicentre (31), randomised, unblinded
- EGDT vs. protocolised care vs. control
- No change in mortality between groups (21% vs. 18% vs 19%)
Steroids:
- The rationale for steroids is that they may:
- C
- Limit ↓ inotropy due to bacterial endotoxin
- ↑ Catecholamine function
- ↓ NO synthetase production
- I
- Correct adrenal insufficiency
- Dampen hyperactive immune response
- C
- CORTICUS (2008)
- 488 adults with severe sepsis
- Multicentre (52), double-blinded, block randomised trial
- Hydrocortisone vs. placebo
- Hydrocortisone
- Hydrocortisone 50mg IV Q6H for 5 days
- Tapered over 6 days
- Placebo
- Hydrocortisone
- Underpowered due to lower-than-expected control group mortality
- Slow recruitment despite large number of sites, suggests selection bias
- No difference in 28 day mortality (39.2% vs 36.1%)
- Secondary outcomes insignificant except faster shock reversal (3.8 vs. 5.8 days) in hydrocortisone group
- ADRENAL (2018)
- 3658 adults with septic shock requiring vasopressors and mechanical ventilation, without various exotic infections
- Multicentre (69), international, double-blinded RCT
- 3800 patients gives 90% power to detect 5% ARR from a baseline mortality of 33%
- Hydrocortisone vs. placebo
- Hydrocortisone
200mg/day via continuous infusion.
- Hydrocortisone
- No difference (27.9% vs. 28.8%) in 90 day mortality
- Secondary outcomes favour steroids:
- ↓ Ventilator days (3 vs. 4 days)
- ↓ Time to shock resolution
- ↓ ICU length of stay (10 vs. 12 days)
- ↓ Blood transfusion (37% vs. 41.7%)
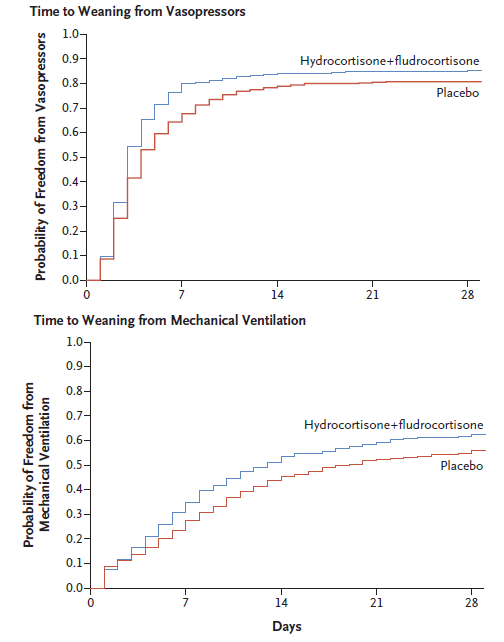
- APROCCHSS (2018)
- 1241 Frenchpersons with probable septic shock for <24 hours, requiring >6 hours vasopressors
- Multicentre (34)
- 320 patients per group to detect 10% ↓ in 90 day mortality at 95% power
- Trial initially also planned to investigate drotrecogin alfa in a factorial design, which required revising trial design after its withdrawal
- Steroids vs. placebo
- Steroids
- Hydrocortisone 50mg IV Q6H
- Fludrocortisone 50μg PO/NG mane
- Both groups had plasma cortisol and short synacthen test performed
- Steroids
- Very high dose of vasopressors at randomisation
- Majority had noradrenaline with mean dose of ~1μg/kg/min
- Several had adrenaline with a mean dose at ~2μg/kg/min (!!)
- Significant ↓ 90 day mortality in intervention group (43% vs. 49%, RR 0.88 (CI 0.78 - 0.99))
- Secondary outcomes broadly favour steroids
Vitamin C:
- The rationale for Vitamin C is that:
- Levels are ↓ in the critically ill
- Oxidative stress is ↑ in the critically ill
- Vitamin C has antioxidant effects that may alleviate some oxidative stress
- Marik et al (2017)
- 47 non-pregnant Americans with severe sepsis or septic shock and pro-calcitonin >2ng/mL
- Single-centre, retrospective, observational study
- Vitamin C 1.5g QID (7 days), hydrocortisone 50mg QID (4 days, then 3 day taper), and thiamine 200mg BD (4 days) vs. standard care
- Significant ↓ in hospital mortality with the “metabolic cocktail” (8.5% vs. 40.4%)
- Prompted a slew of RCTs that reversed this finding
- CITRIS-ALI (2019)
- American English-speaking adults mechanically enilated patients with ARDS and suspected or proven infection with ⩾2 SIRS criteria
- Randomised, double-blinded, placebo-controlled, multi-centre (7)
- Vitamin C (50mg/kg) vs. placebo
- No significant difference in SOFA score, CRP, or thrombomodulin
- Secondary outcomes indicate ↓ 28 day mortality (28% vs 46%), ICU-free days to day 28, and hospital-free days to day 60 in the vitamin C group
- VITAMINS (2020)
- Adults admitted with Septic Shock, not at imminent risk of death
- Multicentre pilot RCT performed based on the now-infamous Marik study
- Randomised to vitamin C (1.5g Q6H), hydrocortisone (50mg Q6H), and thiamine (200mg Q12H) vs. hydrocortisone (50mg Q6H)
- No difference in time alive and free of vasopressors
- Not powered for mortality outcomes
- LOVIT (2022)
- 872 adults with suspected infection requiring ICU admission and vasopressors in Canada, France, and New Zealand
- 50mg/kg Vitamin C vs Placebo
- Significantly ↑ death (44.5% vs 38.5%) and persistent organ dysfunction (9.1% vs 6.9%) in vitamin C group
Other:
- CENSER (2018)
- 456 adults with hypotension and sepsis, without septic shock or other significant acute disease
- Single-centre, blinded RCT
- 300 patients provides 80% power to detect 20% ↑ “shock resolution” at 6 hours, compared to 60% in control group
MAP sustained >65mmHg >15 minutes with >2 hours of UO >0.5mL/kg/hr or ↓ lactate by >10% from initial level. - Noradrenaline vs. placebo
- Noradrenaline
- 0.05μg/kg/min for 24 hours without titration
- D5W placebo
- Open-label vasopressors if MAP <65
- Noradrenaline
- Greater shock resolution at 6 hours in noradrenaline group (76% vs 48%)
- More interestingly, over half of the patients had norad given peripherally and no extravasation injuries occurred
- ANDROMEDA-SHOCK (2019)
- 424 adult south Americans with septic shock requiring vasopressors despite 20mL/kg volume resuscitation
- Multicentre (28), randomised, clinician unblinded, assessor blinded
- 90% power for 15% ARR (!) for mortality
- Peripheral perfusion-targeted resuscitation vs. lactate targeted resuscitation
- Peripheral perfusion
- Finger pad capillary refill time Q30 minutely, then hourly until normalisation
- Targeted capillary refill time <3s
- Lactate targeted
- Lactate measured Q2H for 8 hours
- Targeting ↓ lactate by 20% every 2 hours until normal
- Failure to normalise was targeted with escalating protocols:
- Fluid responsiveness
- PPV or VTI change following passive leg raise
- 500mL crystalloid
- Vasopressor test
- MAP ↑ to 80-85
- If target normalised, MAP maintained
- Otherwise MAP target set to 65
- MAP ↑ to 80-85
- Inodilator test
- Dobutamine or milrinone
- Fluid responsiveness
- Peripheral perfusion
- ↑ Mortality in lactate group (43.4% vs 34.9%)
- Underpowered for outcome
- FABLED (2019)
- Adults in the ED with suspected severe sepsis
- Multi-centre (7) diagnostic cohort study
- 2 sets of blood cultures taken before antibiotics via separate venepuncture
- 1-2 sets of cultures taken 30-240 minutes after antibiotics
- Significant ↓ in positive cultures post antibiotics
- ~30% positive pre-antibiotic, compared to ~20% positive post-antibiotic
RRR ~33%. - More pronounced ↓ in positive post-antibiotic cultures if organism was sensitive to antibiotic used
RRR ~50%. - Positive post-antibiotic cultures were associated with ↑ time to positivity, suggesting ↑ bacterial burden
- ~30% positive pre-antibiotic, compared to ~20% positive post-antibiotic
- CandiSep (2022)
- 342 Adults with sepsis at risk of invasive candidiasis, but without proven fungal infection and not on fungal treatment
- Blood cultures performed and randomised to:
- (1→3)-β-D-Glucan testing performed on blood cultures
Fungal cell wall component that can be detected prior to cultures becoming positive. - Standard cultures
- (1→3)-β-D-Glucan testing performed on blood cultures
- β-D-Glucan group received more (57% vs 27%) and earlier (1.1 vs 4.4 days) antifungal treatment without a change in mortality
- Lower power than anticipated due to lower mortality
- Lower rates of invasive candidiasis than anticipated
- TRISS (2014)
- 998 Scandanavian adults with septic shock and Hb <90g/dL, without active haemorrhage, burns, ACS< previous transfusion, or transfusion reactions
- Multicentre (32), block randomised, stratified by site and haematological malignancy
- 80% power for 9% ARR ↓, assuming 45% control mortality
- Restrictive vs. liberal transfusion
- Restrictive threshold <70g/dL
- Liberal threshold <90g/dL
- Single unit RBC given when threshold met
- No change in 90 day mortality
- Secondary outcomes: ↑ Number of patients and units transfused in liberal group
- Morelli et al (2013)
- 154 β-blocker-naïve Italians with sepsis and a normal CI requiring noradrenaline admitted to a single ICU in Rome
- Single-centre, phase 2, unblinded RCT
- Randomised to esmolol vs. usual care
- Esmolol group had infusion adjusted to achieve HR 80-94
- All patients received:
- PAC
- Hydrocortisone 300mg/day via continuous infusion
- Levosimendan if ↓ DO2 and Hb >80
- CVP and PCWP guided fluid resuscitation and MvO2
- Significant ↓ in HR and mortality in esmolol group, which must be contextualixed against the… high… 80% control group mortality
- High dose vasopressors at randomisation (~0.4μg/kg/min NA), despite normal lactates
- LeoPARDS (2017)
- 516 British adults with suspected sepsis ( by possible infection and ⩾2 SIRS criteria)
- Multicentre (34), double-blind, placebo-controlled RCT
- 90% power to detect a mean difference in SOFA score of 0.5
- Levosimendan vs. standard care
- Levosimendan
0.1µg/kg/min, ↑ to 0.2µg/kg/min at 2-4 hours if tolerated and continued for up to 24 hours. - Placebo
- Levosimendan
- Study drug commenced after adequate fluid resuscitation and restoration of target MAP
- No difference in mean SOFA score, ↑ haemodynamic instability and mean ventilator days in levosimendan group
References
- Bellomo R, Kellum JA, Ronco C et al. Acute kidney injury in sepsis. Intensive Care Med. 2017 Jun;43(6):816-828. doi: 10.1007/s00134-017-4755-7. Epub 2017 Mar 31.
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. New England Journal of Medicine. 2023;388(6):499-510.
- Singer, M., Deutschman, C.S., Seymour, C.W., Shankar-Hari, M., Annane, D., Bauer, M., Bellomo, R., Bernard, G.R., Chiche, J.-D., Coopersmith, C.M., Hotchkiss, R.S., Levy, M.M., Marshall, J.C., Martin, G.S., Opal, S.M., Rubenfeld, G.D., van der Poll, T., Vincent, J.-L., Angus, D.C., 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801.
- Lamontagne F, Masse MH, Menard J, et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N Engl J Med. 2022;386(25):2387-2398.
- Bloos F, Held J, Kluge S, et al. (1→3)-β-d-Glucan-guided antifungal therapy in adults with sepsis: the CandiSep randomized clinical trial. Intensive Care Med. 2022;48(7):865-875. doi:10.1007/s00134-022-06733-x
- Meyhoff TS, Hjortrup PB, Wetterslev J, et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. New England Journal of Medicine. 2022;386(26):2459-2470.
- O’Grady NP, Barie PS, Bartlett JG, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Critical Care Medicine. 2008;36(4):1330.
- Morelli A, Ertmer C, Westphal M, et al. Effect of Heart Rate Control With Esmolol on Hemodynamic and Clinical Outcomes in Patients With Septic Shock: A Randomized Clinical Trial. JAMA. 2013;310(16):1683-1691. doi:10.1001/jama.2013.278477
- Cheng MP, Stenstrom R, Paquette K, et al. Blood Culture Results Before and After Antimicrobial Administration in Patients With Severe Manifestations of Sepsis. Ann Intern Med. 2019;171(8):547-554. doi:10.7326/M19-1696
- Fujii T, Luethi N, Young PJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA. 2020;323(5):423. doi:10.1001/jama.2019.22176
- Fowler AA III, Truwit JD, Hite RD, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322(13):1261-1270. doi:10.1001/jama.2019.11825
- Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA. 2019;321(7):654-664. doi:10.1001/jama.2019.0071
- Annane D, Renault A, Brun-Buisson C, et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. New England Journal of Medicine. 2018;378(9):809-818. doi:10.1056/NEJMoa1705716
- Venkatesh B, Finfer S, Cohen J, et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. New England Journal of Medicine. 2018;378(9):797-808. doi:10.1056/NEJMoa1705835
- Sprung CL, Annane D, Keh D, et al. Hydrocortisone Therapy for Patients with Septic Shock. N Engl J Med. 2008;358(2):111-124. doi:10.1056/NEJMoa071366
- Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. CHEST. 2017;151(6):1229-1238. doi:10.1016/j.chest.2016.11.036
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after Fluid Bolus in African Children with Severe Infection. New England Journal of Medicine. 2011;364(26):2483-2495. doi:10.1056/NEJMoa1101549
- Goal-Directed Resuscitation for Patients with Early Septic Shock. New England Journal of Medicine. 2014;371(16):1496-1506. doi:10.1056/NEJMoa1404380
- Dünser MW, Dankl D, Petros S, Mer M. Clinical Examination Skills in the Adult Critically Ill Patient. Springer International Publishing; 2018.
- Timsit JF, Ling L, De Montmollin E, Bracht H, Conway-Morris A, De Bus L, et al. Antibiotic therapy for severe bacterial infections. Intensive Care Med [Internet]. 2025 Sept 1.