Blood Products
Therapeutic substances derived from human blood, including:
- Fractionated products
- Packed cells
- Clotting factors
- Fresh frozen plasma
- Factor concentrates
- Cryoprecipitate
- Fibrinogen concentrate
- Platelets
- Granulocytes
- Whole blood
Packed Cells
Donor blood is cytapheresed to produce cells and a small volume of carrier plasma. PRBC:
| Recipient Group | Compatible Donor |
|---|---|
| Unknown | O |
| O | O |
| A | A O |
| B | B O |
| AB | AB A or B O |
| Notes | Rhesus negative cells should be used for pre-menopausal females until the blood group is established. |
- 1 unit ≈ 200-300mL
Generally raises Hb by ~10g/dL. - Are not fully functional
Develop storage lesions after donation, rendering them progressively less effective:- ↓ O2 offloading at tissues which impedes oxygen extraction
- ↓ Cell deformability
↓ ATP reduces cell membrane maintenance, resulting in loss of biconcave disc shape. This causes:- ↓ Microvascular flow
- ↓ Functional capillary density
- ↑ Fragility
↑ Risk of haemolysis and reticuloendothelial destruction.- Conjugated bilirubinaemia
- Accumulation of metabolic products
- ↑ Neutrophil activation
- Are generally routinely leukcoreduced
Removal of granulocytes and leukocytes prior to storage which:- ↓ Transfusion reactions
TRALI, febrile reactions, CMV infection, transfusion-associated GvHD, alloimmunisation. - ↓ Storage lesions
- ↓ Transfusion reactions
- Transfusion should:
- Begin once removed from refrigerator
- Complete within 4 hours of removal
- Returned to controlled storage within 30 minutes of removal (if not transfused)
- Cannot be returned if out of controlled storage for >30 minutes
The small amount of incompatible plasma that is transfused with unmatched (e.g. O-) RBCs is not considered to be clinically significant.

Fresh Frozen Plasma
Donor plasma remaining after cytapheresis of RBC, which is then frozen and re-thawed prior to use. FFP:
| Recipient Group | Compatible Donor |
|---|---|
| Unknown | AB Low anti-B A. |
| O | O A B AB |
| A | A AB Low anti-A B |
| B | B AB Low anti-B A |
| AB | AB Low anti-B A Low anti-A B |
- 1 unit ≈ 200-300 ml
Contains normal levels of clotting factors, albumin, and immunoglobulin:- ~0.5g of fibrinogen
- 0.7 IU/mL of Factor VIKI
- Dose is usually 10-15ml/kg
This is a substantial volume for a normovolaemic patient - consider factor concentrates. - Stored at -18°C to prevent clotting factor degradation
Must be thawed prior to administration. - ABO compatibility highly desirable but not essential
- Low-titre anti-A and anti-B are preferable when transfusing ABO incompatible plasma
- Transfusion should:
- Commence within 30 minutes
- Be completed within 4 hours of thawing
- Cannot be returned after 30 minutes out of controlled storage
- Cannot be re-frozen
- If returned within 30 minutes, it can be refrigerated for up to 24 hours at 2-6°C and re-issued
FFP should not be used as a volume expander for hypovolaemia in absence of an appropriate indication.
Prothrombin Complex Concentrates
Freeze-dried preparation from donor plasma that:
3-factor PCC is also known as factor IX complex.
- Contains the vitamin-K dependent clotting factors
- Factor II
- Factor VII
Not present in all preparations; so these are usually described as either:- 4-factor PCC
Contain all factors. - 3-factor PCC
Contain II, IX, and X.
- 4-factor PCC
- Factor IX
- Factor X
- Is indicated for:
- Correction of vitamin K antagonism
- Correction of coagulopathy in absence of hypovolaemia
I find PCC useful in the operating room for rapidly correcting coagulopathy, as it can be given far more quickly than an equivalent factor-load of FFP.
Single-Factor Concentrates
Single factors are produced either through isolation of donor plasma isolated or recombinant production. Available factors include:
- Antithrombin III
Treatment of heparin resistance:- For CPB: 1000-2000 units if inadequate ACT after >600 U/kg of UFH
- Activated Factor VII
- Vitamin-K dependent clotting factor that enhances haemostasis at site of injury without generalised hypercoagulability
- Clots formed are stronger and more resistant to fibrionlysis than normal clot
- Risk of generalised hypercoagulable state
- Indicated for congenital factor VII deficiency, or inhibitors to VIII or IX, but the majority of use is off-label from refractory surgical or traumatic haemorrhage
- Caution in patients with history of thromboembolic events
- Consider when:
- Fibrinogen >0.5g/dL
- Platelets >50×109/L
- pH >7.1
- Dose variable
- 20μg/kg increments in semi-controlled situations
- 90-120μg/kg for exsanguinating trauma
- Vitamin-K dependent clotting factor that enhances haemostasis at site of injury without generalised hypercoagulability
- Factor VIII
- Heat treated to inactivate HIV
- For treatment of haemophilia A
- Factor IX
Activated Factor VII is also known as recombinant activated coagulation factor VII, FVIIa, rFVIIa, eptacog alfa, and NovoSeven.
Cryoprecipitate
FFP derivative which:
Cryoprecipitate is manufactured by freezing and then thawing FFP, collecting the precipitate, and then re-suspending it in plasma.
- May be presented as:
- Single donor
30-40mL. - Apheresis
~50mL.
- Single donor
- Stored at -18°C
- Contains concentrated:
- Fibrinogen
Main advantage, as there is little fibrinogen in FFP. - Factor VIII
- Factor XIII
- Fibronectin
- vWF
- Fibrinogen
- Transfusion should:
- Commence within 30 minutes
- Be completed within 4 hours
- Cannot be returned after 30 minutes out of controlled storage
Fibrinogen Concentrate
Freeze-dried human plasma:
- Contains ~1g of fibrinogen
- Reconstituted with water
- Used in massive transfusion
Platelets
- 1 unit ≈ 50mL
Generally raises platelet count by ~10× 109/L. - Usually come in ~200mL, 4-unit bags
These may be:- Pooled from whole blood donation from separate donors
- Single-donor, collected via apheresis
- ABO match is preferable but not essential
- Apheresis platelets have a lower titre of anti-A and anti-B and have a lower risk of haemolysis
These are preferred when ↓ exposure of the recipient to multiple donors is desirable.
- Apheresis platelets have a lower titre of anti-A and anti-B and have a lower risk of haemolysis
- Stored at 20-24°C with constant agitation
- Transfusion should:
- Commence as soon as received
- Completed within 1 hour of issue
- May be able to be returned after 1 hour, conditional on storage
| Recipient Group | Compatible Donor |
|---|---|
| Unknown | O or apheresis low-antigen A |
| O | O Apheresis low-antigen A B |
| A | A Apheresis B or O |
| B | B Apheresis low A-antigen A, A, or O AB |
| AB | AB Apheresis low anti-B A or anti-A B Apheresis low anti-A/anti-B O |
The volume of incompatible plasma administered with platelets is significantly higher (due to greater platelet volume), but because of greater limitations in platelet availability the higher risk is assumed necessary.
Granulocytes
Granulocyte (neutrophils) are harvested from the buffy coat and:
- Indicated for neutropenic sepsis
- Must be ABO and Rh compatible
- Irradiated prior to transfusion
Whole Blood
Whole blood:
1:1:1 mixtures of fractionated products do not faithfully reconstitute whole blood, but instead yeild a dilute mixture with:
- Haematocrit 29%
- Platelet count ~90× 109/L
- Has full haemostatic function
Preferable to fractionated products for treatment of massive haemorrhage, when available. - Has an ↑ complexity of type matching
Options include:- Group-specific whole blood transfusion
Transfuse from donor with identical ABO group. - Low-titre type-O whole blood transfusion
From O donor with low anti-A or anti-B levels.- This ↑↑ complexity of determining the patients blood group
- Group should be taken prior to administering low-titre whole blood
- Standard of care in WWII, as well as the wars in Korea and Vietnam
- Group-specific whole blood transfusion
- Collected from donors into citrated bags
- Goal is generally ~450mL/585g
- Bag should be agitated periodically to ↓ risk of clot formation
- Is divided into:
- Fresh whole blood
- Stored at room temperature and administered within 24 hours
- Generally not tested for transfusion-transmitted diseases and so not appropriate for civilian use
- Refrigerated within 8 hours and stored
- Stored whole blood
- Can be fully tested for transfusion-transmitted disease
- Can be kept for:
- 21 days in CPD (Citrate Phosphate Dextrose)
- 35 days in CPDA-1 (Citrate-phosphate-dextrose adenine)
- Fresh whole blood
Irradiation of Blood Products
Exposure of donated cellular products to high (25Gy) doses of ionised radiation:
- Destroys contained lymphocytes
- ↓ Risk of transfusion-associated GvHD
- Does not make the blood radioactive, and is not otherwise damaging
- Some units may routinely irradiate all cellular products
- Is not required for non-cellular (e.g. plasma) products
- Is indicated for transfusion to patients who are:
- Immunocompromised
- Lymphoma
- Stem-cell transplants
- Aplastic anaemia
- Related-donor transfusion
- Granulocyte transfusions
- HLA-matched platelets
- Neonates
- Immunocompromised
Key Studies
Transfusion Thresholds:
- TRICC (1999)
- 838 anaemic (Hb <90g/dL) Canadians with >24 hour expected ICU stay, without active bleeding, chronic anaemia, or post-cardiac surgery
- Multicentre, non-blinded, randomised trial
- 1620 patients provides 95% power for a 27.5% RRR from a liberal transfusion mortality of 27%
- Restrictive vs. liberal transfusion
- Restrictive
Transfusion threshold: Hb <70g/dL - Liberal
Transfusion threshold: <100g/dL
- Restrictive
- No change in 30 day mortality (18.7% vs. 23.3%)
- Secondary outcomes: Higher hospital mortality in liberal group
- Grossly underpowered without explanation as to why
- TRISS (2014)
- 998 Scandanavian adults with septic shock and Hb <90g/dL, without active haemorrhage, burns, ACS< previous transfusion, or transfusion reactions
- Multicentre (32), block randomised, stratified by site and haematological malignancy
- 80% power for 9% ARR ↓, assuming 45% control mortality
- Restrictive vs. liberal transfusion
- Restrictive threshold <70g/dL
- Liberal threshold <90g/dL
- Single unit RBC given when threshold met
- No change in 90 day mortality
- Secondary outcomes: ↑ Number of patients and units transfused in liberal group
- TRICS-III (2017)
- 5092 non-pregnant, non-lactating, adult standard cardiac surgical patients (not heart transplants or VAD) with EUROSCORE I >6
- Randomised, allocation concealed, assessor blinded, multicentre (73), international RCT
- 90% power for 3% non-inferiority margin for composite of death, MI, inpatient RRT
- Restricted vs. liberal transfusion
- Restrictive
Transfused if Hb <75g/dL. - Liveral
Transfused if Hb <95g/dL intraoperatively or in ICU, or <85g/dL once discharged from ICU.
- Restrictive
- No difference in primary outcome (11.4% vs. 12.5%), or any secondary outcomes
- Subgroup showed restrictive transfusion ↓ primary outcome in >75 year olds
- ~50% of the liberal group were transfused an average of 2 units, compared to 7~3% of the liberal group (3 units)
Red Cell Youth:
- Historically, the oldest available cells have been used to avoid wastage
- Critically ill patients (who are more susceptible to storage lesions, and receive more blood transfusions) may benefit from receiving younger product
- TRANSFUSE (2017)
- Non-pregnant adults requiring transfusion, without a previous blood transfusion, haematological disease, transplant, or after cardiac surgery
Cardiac surgery excluded due to low mortality. - Multicentre (59), double-blinded, allocation-concealed, RCT
- 90% power to detect 4.2% ↓ in 90 day mortality, assuming 28% control mortality
- Freshest vs. oldest blood
- Fresh
- Mean storage 11.8 +/- 5.3 days
- Old
- Mean storage 22.4 +/- 7.5 days
- Fresh
- No mortality difference (24.8% vs. 24.1%)
- Secondary outcomes showed no difference except ↑ febrile non-haemolytic reactions in fresh group
- This is the definitive trial on this topic
- Non-pregnant adults requiring transfusion, without a previous blood transfusion, haematological disease, transplant, or after cardiac surgery
Viscoelastic Testing:
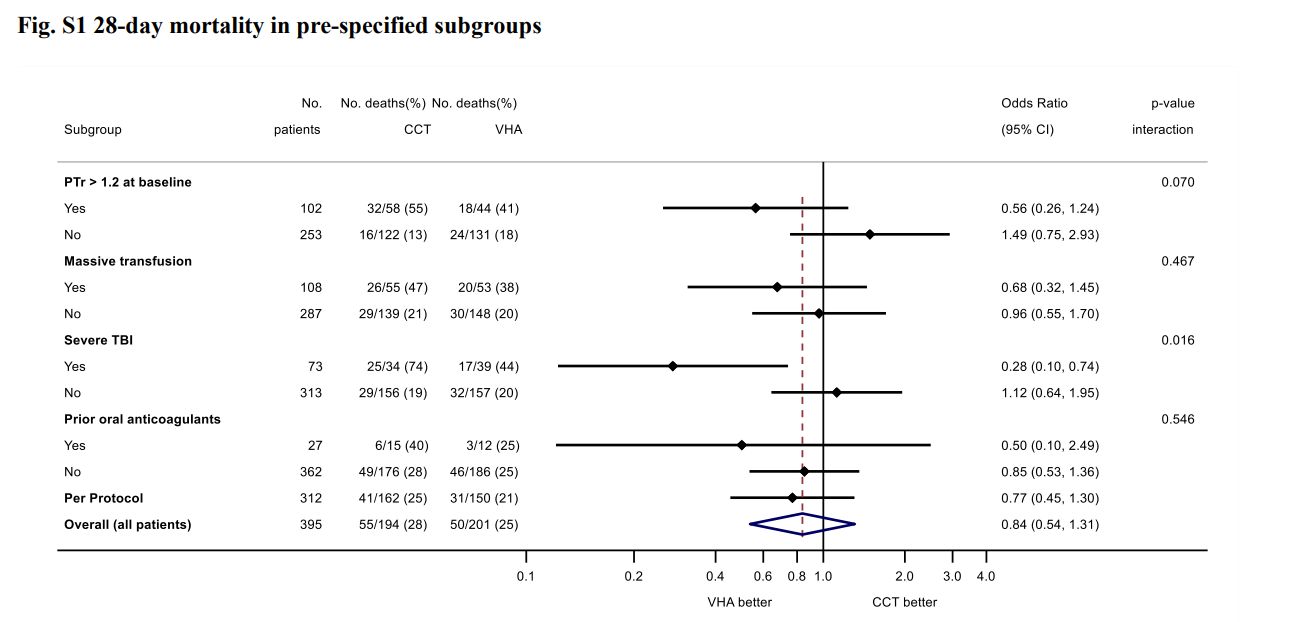
- ITACTIC (2020)
- 392 adult Europeans with trauma requiring MTP activation and active bleeding within 1 hour of ED admission and 3 hours of injury
- Block randomised multicentre (7), clinician unblinded, researcher blinded RCT
- 392 patients provides 80% for 15% ↓ in death or massive transfusion at 24 hours
- Viscoelastic assays (ROTEM or TEG) vs. conventional coagulation testing
- Protocolised treatment of coagulation abnormalities for both groups
- All patients received 1:1:1 transfusion, with algorithmic administration of TXA, fibrinogen, and additiojnal products
- Most patients severely injured
- No difference in primary outcome (67% vs 64%)
Ambitious difference for a monitoring trial. - Secondary outcome supports 28 day mortality ↓ in TBI group
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Yazer MH, Waters JH, Spinella PC, et al. Use of Uncrossmatched Erythrocytes in Emergency Bleeding Situations. Anesthesiology. 2018;128(3):650-656.
- Berezina TL, Zaets SB, Morgan C, et al. Influence of Storage on Red Blood Cell Rheological Properties. Journal of Surgical Research. 2002;102(1):6-12. doi:10.1006/jsre.2001.6306
- NZ Blood Service. Transfusion Medicine Handbook, 3rd Edition. Accessed June 8, 2023.
- National Blood Authority. Patient Blood Management Guidelines: Module 1 - Critical Bleeding and Massive Transfusion. National Health and Medical Research Council; 2011. Accessed June 8, 2023.
- Cap A, Beckett LA, Benov MA, et al. Whole Blood Transfusion. Joint Trauma System Clinical Practice Guideline. 2018.
- Cap AP, Beckett A, Benov A, et al. Whole Blood Transfusion. Military Medicine. 2018;183(suppl_2):44-51. doi:10.1093/milmed/usy120
- Baksaas-Aasen K, Gall LS, Stensballe J, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2021;47(1):49-59. doi:10.1007/s00134-020-06266-1
- Mazer CD, Whitlock RP, Fergusson DA, et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. New England Journal of Medicine. 2017;377(22):2133-2144. doi:10.1056/NEJMoa1711818
- Holst LB, Haase N, Wetterslev J, et al. Lower versus Higher Hemoglobin Threshold for Transfusion in Septic Shock. N Engl J Med. 2014;371(15):1381-1391. doi:10.1056/NEJMoa1406617
- Cooper DJ, McQuilten ZK, Nichol A, et al. Age of Red Cells for Transfusion and Outcomes in Critically Ill Adults. New England Journal of Medicine. 2017;377(19):1858-1867. doi:10.1056/NEJMoa1707572
- Hébert PC, Martin C, Yetisir E. A Multicenter, Randomized, Controlled Clinical Trial of Transfusion Requirements in Critical Care. The New England Journal of Medicine. Published online 1999.
- Martinowitz U, Michaelson M, Force TIM rFVIIa T. Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli Multidisciplinary rFVIIa Task Force. Journal of Thrombosis and Haemostasis. 2005;3(4):640-648. doi:10.1111/j.1538-7836.2005.01203.x