Critical Bleeding
Critical bleeding is life-threatening bleeding that is likely to require massive transfusion.
This discusses critical bleeding a general approach to haemostatic resuscitation. Damage control surgery and the particulars of major haemorrhage in the trauma patient are covered under Damage Control Surgery.
Critical bleeding is also defined as bleeding in a critical area (e.g. intracranial, intraocular, intraspinal), resulting in morbidity or mortality. Whilst an epidural haematoma is both a) bleeding and b) critical, the management principles are so different from the exsanguinating patient that I’ve sidelined this definition.
Massive transfusion is defined as one of:
- Whole blood volume in 24 hours
- 10 PRBC in 24 hours
Functionally the whole blood cell volume in the 70kg-Guyton man. - ⩾50% of blood volume in 4 hours
>40mL/kg in children.
These definitions are notably all retrospective, and so remain impractical for clinical use.
Epidemiology and Risk Factors
Principles of haemostatic resuscitation are derived from the following:
- 40% of traumatic deaths in the first 24 hours occur due to haemorrhage
- 25% of severe trauma patients are coagulopathic on arrival
- Coagulopathy is a very poor prognostic sign
3× mortality in coagulopathic patients. - Acidosis significantly impairs both haemostatic and haemodynamic function
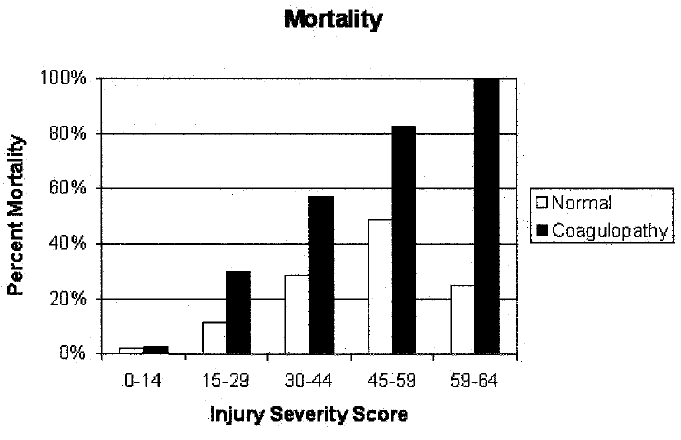
Predicting massive transfusion:
- Assessment of Blood Consumption (ABC Score)
⩾2 factors suggests 85% likelihood of MTP.- HR ⩾120
- SBP ⩽90
- Penetrating injury
- Positive FAST scan
- Injury patterns
- Uncontrolled:
- Truncal bleeding
- Junctional haemorrhage
- Large soft tissue bleeding
- Amputations
- Mangled extremities
- Clinical coagulopathy
- Severe hypothermia
- Uncontrolled:
- COAST score
Note that the haemoglobin is not an indicator for MTP activation, as it is a measure of concentration and does not reliably change during acute haemorrhage.
| Variable | Score |
|---|---|
| Entrapment |
|
| SBP |
|
| Temperature |
|
| Suspected abdominal trauma |
|
| Suspected abdominal/pelvic trauma |
|
Pathophysiology
Coagulopathy is multifactorial; key contributors include:
- Shock
- Ischaemia
- Acidosis
- Tissue injury
- Hypothermia
- Haemodilution
- Crystalloid resuscitation
- Unbalanced transfusion
- Consumption of clotting factors
- ↓ Platelet function
- Hyperfibrinolysis
- Dysfibrinogenaemia
Secretion of functionally abnormal fibrinogen due to autosomal dominant defect.
Aetiology
Clinical Features
Assessment
History:
Exam:
Investigations
Massive transfusion is characterised by bleeding faster than the time coagulation assays take to run. Consequently:
- Most investigations are of limited benefit as decisions have to be made (and the clinical situation changed) prior to tests returning
Despite this, they should be checked every thirty minutes to monitor trajectory of resuscitation. - Protocolisation of MTP ↓ this uncertainty
- Once haemorrhage has slowed to a point that coagulation assays accurately reflect the current physiological state, individualised approaches can be used
Bedside:
- Point-of-care coagulation assays
- ABG
- Calcium
- Hb
Laboratory:
- Coagulation
- APTT
- PT
- Fibrinogen
Imaging:
Other:
Diagnostic Approach and DDx
Management
The principles of haemostatic resuscitation are:
- Stop the bleeding
- Permissive hypotension
- Early haemorrhage control
Early surgery or interventional radiology.
- Correction of physiological abnormalities
The lethal triad:- Acidosis
- Hypothermia
- Coagulopathy
- Massive transfusion
Early notification to the blood bank.
This emphasises management of blood products and physiological state, particulars of haemorrhage control and permissive hypotension are better covered under Damage Control Surgery.
Resuscitation:
Note that 1 bag of platelets contains ~4 units in Australia and New Zealand, so that 4 bags of PRBC and FFP should be given for each bag of platelets.
- B
- CO2 control
For acidosis; balanced by other injury concerns.
- CO2 control
- C
- Permissive hypotension
- Balanced transfusion
- 1:1:1 transfusion
- Three units of cryoprecipitate should be given for every 4 units of PRBC
- Additional cryoprecipitate should be given if fibrinogen is <1
- Additional unit of platelets should be given if platelets are <75×109/L
- Three units of cryoprecipitate should be given for every 4 units of PRBC
- What products should be transfused should ideally be determined by the blood bank
- 1:1:1 transfusion
- Avoid crystalloid
- Permissive hypotension
- E
- Temperature >36°C
- External warming
- Warm all transfuse blood products
- Temperature >36°C
- F
- Correct acidosis
- Calcium
Target ionised calcium >1mmol/L.
- H
- TXA
1g over 10 minutes. - Consider recombinant factor VIIa
- TXA
Different blood banks may have different protocols for sending blood packs:
- Continue to send until requested to stop
- Each pack made ready, and sent on request
In addition to the regular resuscitation roles, the MTP requires additional allocation of roles:
- MTP activator and lead
- Lab monitor
- Blood preparation and administration
2 people for checking. - Runner
- Scribe
Specific therapy:
- Pharmacological
- Procedural
- Physical
Supportive care:
Disposition:
Endpoints
Goals indicating adequate resuscitation and haemostasis are:
- No evidence of further haemorrhage
- C
- SBP 80-90mmHg
- MAP >50mmHg
- E
- Temperature >35°C
- F
- pH >7.3
- iCa2+ >1.1mmol/L
- H
- Hb >70g/L
- INR <1.5
- APTT <40s
- Fibrinogen >2g/dL
- Platelet >50-100×109/L
Higher target if head injury or intracranial or intraspinal haemorrhage.
Marginal and Ineffective Therapies
Anaesthetic Considerations
Complications
Complications specific to massive transfusion include:
General complications of blood transfusion are covered under Transfusion Reactions.
- Blood product related
Due to the large amount of blood product infused.- Citrate toxicity
Citrate used to anticoagulate blood products (particularly PRBC) will chelate ionised calcium and magnesium, which may contribute to ↓ inotropy and (if severe) coagulopathy. - Acid load
Stored blood contributes to metabolic acidosis which may worsen coagulopathy. Contributing acids include:- Citric acid
From citrate. - Lactic acid
From RBC metabolism.
- Citric acid
- Hyperkalaemia
Higher potassium load in PRBC may ↑ serum potassium in massive transfusion scenarios. - Hypokalaemia
Late and relatively rare complication occurring due to potassium uptake by transfused RBCs, which occurs as they correct electrolyte composition. - Hyperbilirubinaemia
- Haemolysis of transfused cells will ↑ unconjugated bilirubin
- Conjugated bilirubin may ↑ if liver excretion is impaired
e.g. Due to ischaemic liver injury.
- Citrate toxicity
- Procedural
- Hypothermia
Administration of non-warmed blood products has several detrimental effects:- Left-shift of oxyhaemoglobin-dissociation curve
- ↓ RBC deformity
- ↑ Serum K+
- ↑ Metabolic demand
Warming from 4°C → 37°C requires 1255kJ and ~62L of oxygen.
- Air embolism
- Due to rapid infusion in pressurised circuits
Modern devices contain bubble detectors which ↓ this risk, although cases are reported when using other equipment (e.g. arterial line pressure bags).
- Due to rapid infusion in pressurised circuits
- Hypothermia
The acid and citrate load are ↓ with citrate-phosphate-dextrose blood products.
Prognosis
Key Studies
Antifibrinolytics:
- TXA inhibits plasminogen activation (and at higher doses, directly inhibits plasmin), ↓ fibrinolysis
- Exsanguination is a major cause of traumatic death, and is exacerbated by hyperfibrinolysis of traumatic coagulopathy
- CRASH-2 (2010)
- 20,211 trauma patients worldwide
- TXA vs. placebo
- 1g TXA in first 3 hours followed by 1g infusion over 8 hours
- Small, significant ↓ in all-cause and bleeding mortality
- Better outcomes if TXA within 1 hour of injury
- Cost-beneficial
- Concern that majority of patients were in LMIC, ↓ generalisability to systems with significant healthcare resources
- PATCH (2023)
- Non-pregnant, non-nursing-home-resident Australasian or German adults, with major trauma and COAST score >3
- 90% power to detect 9% absolute ↑ in favourable GOS-E
- Blinded, allocation concealed, RCT; stratified by jurisdiction and GCS <9
- TXA vs. placebo
- TXA group
- 1g over 10 minutes at scene or en route to hospital
- 1g over 8 hours
- TXA group
- No difference in survival with favourable functional outcome (53.7% vs. 53.5%)
- Significantly ↓ mortality in TXA group (9.7% vs. 14.1%)
- No difference in DVT incidence
- Significant loss to followup (14%) and protcol deviations
- 33% of TXA group; 16% received open-label TXA and 21% did not receive 2nd dose
- 37% of placebo group, 17% of whom received open-label TXA and 25% did not receive 2nd dose
| Variable | Score |
|---|---|
| Entrapment |
|
| SBP |
|
| Temperature |
|
| Suspected abdominal trauma |
|
| Suspected abdominal/pelvic trauma |
|
The Glasgow Outcome Scale - Extended (GOS-E) is commonly used to measure TBI outcome:
- Death
- Vegetative state
Unaware, periods of spontaneous eye opening, persistent reflex responses. - Lower severe disability
Dependent, unable to be left alone for >8 hours. - Upper severe disability
Dependent, able to be left alone for >8 hours. - Lower moderate disability
Independent at home, dependent outside, unable to return to work. - Upper moderate disability
Independent at home, dependent outside, able to return to work with special arrangement. - Lower good recovery
Some disability but able to return to work. - Upper good recovery
Some deficits but not disabling.
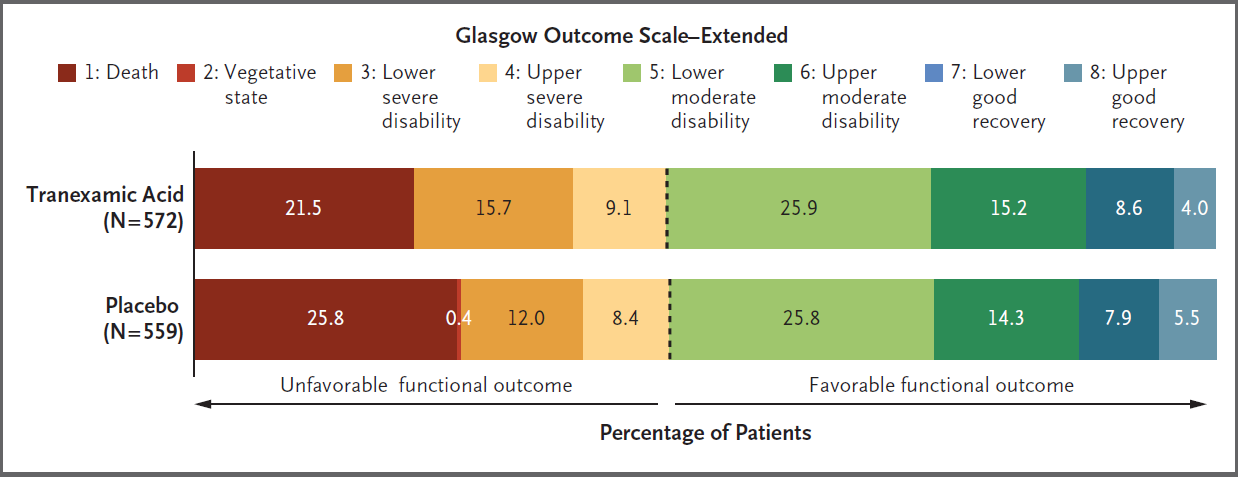
Transfusion ratios:
- PROPPR (2015)
- 680 North American trauma patients >50kg/15 years at 12 Level 1 centres randomised to 1:1:1 vs. 1:1:2 transfusion
- 1:1:1 consisted of equal parts platelets, FFP, and RBC
- 1:1:2 consisted of equal parts of platelets and FFP, with 2 units of RBC
- Improved haemostasis at 24 hours in the 1:1:1 group
- No difference in 24 hour or 30 mortality
- Underpowered for observed mortality difference
- High prevalence (30+%) penetrating trauma
- 680 North American trauma patients >50kg/15 years at 12 Level 1 centres randomised to 1:1:1 vs. 1:1:2 transfusion
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- NZ Blood Service. Transfusion Medicine Handbook, 3rd Edition. Accessed June 8, 2023.
- National Blood Authority. Patient Blood Management Guidelines: Module 1 - Critical Bleeding and Massive Transfusion. National Health and Medical Research Council; 2011. Accessed June 8, 2023.
- Brohi K, Singh J, Heron M, Coats T. Acute Traumatic Coagulopathy: The Journal of Trauma: Injury, Infection, and Critical Care. 2003;54(6):1127-1130.
- Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. The Lancet. 2010;376(9734):23-32.
- Transfusion of Plasma, Platelets, and Red Blood Cells in a 1:1:1 vs a 1:1:2 Ratio and Mortality in Patients With Severe Trauma: The PROPPR Randomized Clinical Trial. Bleeding and Transfusion. JAMA. JAMA Network. Accessed June 8, 2023.