COVID-19
Also known as coronavirus disease 2019, COVID, and SARS-CoV-2.
COVID-19 is a respiratory viral disease caused by the SARS-CoV-2 beta-coronavirus that:
- Has an incubation period of 4-5 days
- Becomes symptomatic between 2-7 days
In the majority (75%) of cases; and rarely after 14 days. - May spread from asymptomatic patients
Up to 50%. - Has a broad spectrum of clinical presentation:
- Mild URTI symptoms in ~80% for ~7 days
- Respiratory failure requiring hospital admission in ~15%
Generally between day 5-10. - Severe pneumonitis and ARDS or multiorgan failure in ~5%
- Mild URTI symptoms in ~80% for ~7 days
Epidemiology and Risk Factors
Pandemic:
- Disease first identified in Wuhan City, Hubei in November 2019
- Global emergency declared 30 January 2020
- Global pandemic declared 11 March 2020
Transmission:
- Person-to-person
- Via respiratory droplets, contact, and possible aerosols
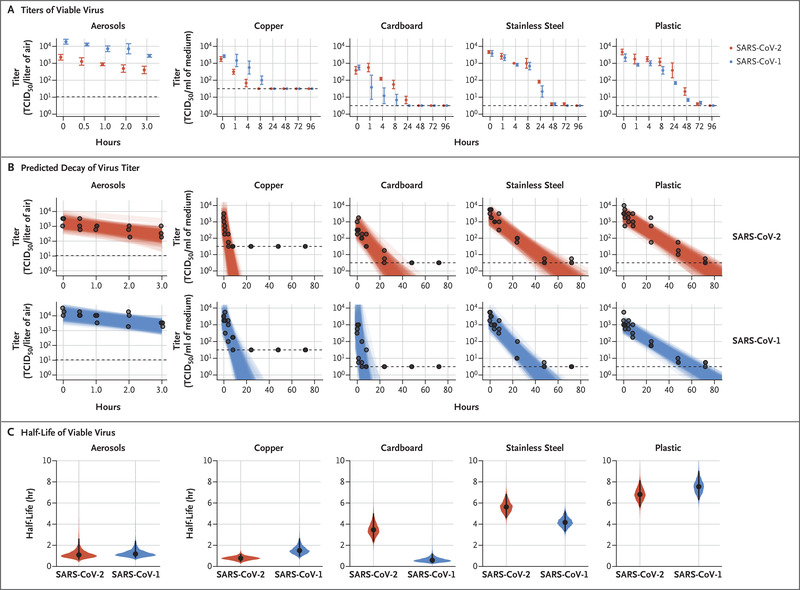
- Droplets may persist for up to 4 days
Pathophysiology
Viral characteristics:
- Cellular entry effected via trans-membrane glycoproteins (“spike”) proteins
- Bind to surface ACE2 receptors
Found on:- Pneumocytes
- Respiratory tract epithelium
- Small bowel enterocyte
- Bind to surface ACE2 receptors
- Hypoxaemia with normal lung compliance
- Diffuse alveolar damage
Coronaviruses are named due to their characteristic halo under electron microscopy, and include:
- Severe Acute Respiratory Syndrome
SARS-CoV, in 2002. - Middle East Respiratory Syndrome
MERS-CoV, in 2012.
Staging:
- Early Infection
- Non-specific clinical features
- Lymphopenia and pro-coagulant state
- Pulmonary phase
- Dyspnoea and hypoxaemic respiratory failure
- Abnormal chest imaging
- Hyper-inflammation phase
- ARDS
- Shock
- ↑ Inflammatory markers
Clinical Features
Features are non-specific, and include:
- Fever
80-90%; typically high and persistent. - Dry cough
60-70%. - Fatigue
40%. - Dyspnoea
Typically around day 6. - Myalgias
- Sore throat
- Headache
- Chills
- Nausea/vomiting
- Anosmia
~30%.
Hypoxaemia may occur without breathlessness.
A substantial proportion (17-30%) of infected may never develop symptoms.
Assessment
| Severity | Characteristics |
|---|---|
| Mild | No features suggestive of a complicated course:
|
| Moderate | Stable but with evidence of lower respiratory disease, such as:
|
| Severe | Either:
|
| Critical | Any of:
|
*Or above 90% for patients with chronic lung disease.
The PaO2:FiO2 (P:F) and SpO2:FiO2 and (S:F) ratios quantify the degree of hypoxia relative to the degree of oxygen supplementation.
They are calculated as:
- \(P/F = {PaO_2 \over FiO_2}\)
- \(S/F = {SpO_2 \over FiO_2} \times 1000\)
Where:
- \(PaO_2\) is in mmHg
- \(FiO_2\) is a decimal (0-1)
History
Exam
Investigations
Bedside:
- Rapid antigen testing
Laboratory:
- Blood
- FBE
- Leukopaenia or leukocytosis may occur
Typically ↑ WCC in critically unwell. - Lymphopaenia
- Thrombocytopaenia
- Leukopaenia or leukocytosis may occur
- LFT
Monitor when using immunosuppressive agents, and consider ceasing if ↑ transaminases. - ↑ LDH
- Coagulation studies
- DIC
- Antibody testing
IgM or IgG antibodies:- Require up to 14 days to become positive
- Detect presence of recent infection
- Inflammatory markers
Typically correlate with severity. Include:- CRP
Rate of rise correlates with degree of inflammation and is a marker of future clinical deterioration. - Procalcitonin
- IL-6
- CRP
- FBE
- Respiratory sample
- PCR
- Sensitivity 60-95%
Varies depending on quality of sample and amount of viral shedding. - The cycle time (or cycle threshold) is number of PCR cycles that must be performed before a result becomes positive
- Provides an estimate of the viral load in the sample
A lower cycle threshold indicates a higher viral load. - Can indicate the infectiveness of the patient
- Does not correlate with severity of disease
- The threshold for a significant cycle time varies depending on the test used
- Provides an estimate of the viral load in the sample
- Sensitivity 60-95%
- PCR
Bronchoalveolar lavage is more sensitive, but is aerosol generating.
Imaging:
Radiological changes develop between 2-5 days, and become maximal at day 13-15.
- CXR
- Bilateral alveolar opacities most common
- Pleural effusions are uncommon
- CT chest
- Routine CT is not required
- Typical appearance includes:
- Peripheral, bilateral ground glass opacities
- Multifocal ground glass opacities
- Organising pneumonia in late disease
- Non-specific features include:
- Multifocal, diffuse, or unilateral ground glass opacitie
- Indicating for investigating:
- PE
- Non-resolving disease for investigation of persistent pneumonic causes
Other:
Diagnostic Approach and DDx
Diagnostic criteria change overtime, however:
- Confirmed case
Positive:- NAA
- Viral culture
- Probable case
All of:- Not tested
- Febrile (>38°C) or symptomatic
- Household contact of confirmed or probable case
- Suspected case
Meets both clinical and epidemiological criteria:- Clinical
Febrile or symptomatic. - Epidemiological
Significant risk factor in the last 14 days:- Close contact with confirmed or probable case
- Travel
- Cruise ship exposure
- Healthcare exposure
- High-risk community exposure
- Clinical
Management
- Airborne precautions
- Use appropriate PPE
- Manage hypoxaemia
- Prone positioning
- Non-invasive ventilation is preferred to invasive ventilation where possible
- Invasive ventilation should use a lung protective strategy
- Immunosuppression
Choice varies depending on disease severity.
| Severity | Immunosuppression Used |
|---|---|
| Mild |
|
| Moderate |
|
| Severe |
|
Resuscitation:
- A
- Intubation
Consider:- Aerosoliation
- Rapid hypoxaemia on induction
- Hypotension post-induction
- Hypovolaemia
- Effect of high PEEP
- Intubation
- B
- Correct hypoxaemia to SpO2 92-96%
- High flow nasal oxygen
- Titrate FiO2 to SpO2
- Titrate flow to work of breathing
- Minimal effect on recruitment
Combine with proning to minimise atelectasis.
- NIV
- CPAP up to 15cmH2O
- Assists recruitment and may be more beneficial in early disease
- Significantly ↓ Rate of intubation
- Invasive ventilation
- Permissive hypercapnoea
- High flow nasal oxygen
- Proning
- Correct hypoxaemia to SpO2 92-96%
Specific therapy:
- Pharmacological
- Remdesivir 200mg IV load, then 100mg IV OD for a total of 5 days
- Paediatric dosing: 5mg/kg load, then 2.5mg/kg on subsequent days
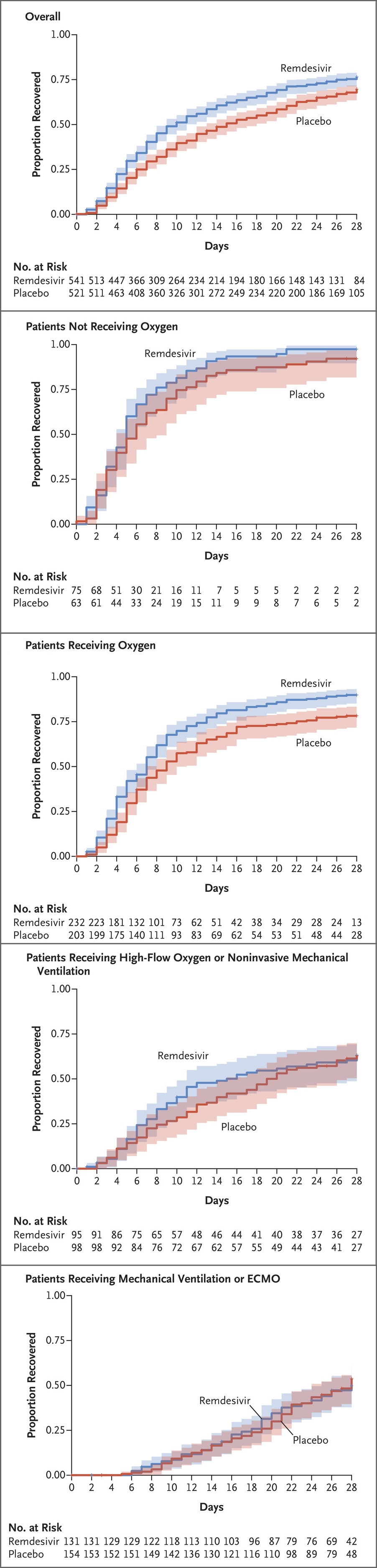
- Indicated only for mild and moderate disease, within 7 days of symptom onset
Limited utility in the hospital population. - ↓ Time to clinical recovery in mild cases
- Contraindicated with:
- Renal failure
- Multi-organ failure
- Neutropenia
- Dexamethasone 6mg IV/PO daily for at least 10 days
- Indicated for severe or critical disease
- Methylprednisolone 32mg/day or prednisolone 40mg/day may be used if dexamethasone is unavailable
- Consider ↑ to 12-20mg/day if tocilizumab or baricitinib are not available or are contraindicated
- Significant ↓ in absolute mortality
- Baricitinib 4mg PO daily for up to 14 days, or until hospital discharge
- Janus kinase inhibitor, ↓ downstream effects of IL-6
- Significant ↓ mortality in severe disease
- Use 2mg PO daily if eGFR <30mL/min
- Contraindicated in:
- ESRD
- Acute severe infection
- Severe immune dysfunction
- Neutropenia
- Pregnancy
- Tocilizumab 8mg/kg single dose
- Indicated for patients requiring intubation and who have not received baricitinib
- ↓ Mortality in combination with dexamethasone
- Sarilumab may be used if tocilizumab is unavailable
- Remdesivir 200mg IV load, then 100mg IV OD for a total of 5 days
- Procedural
- VV ECMO
- Physical
- Proning
For all patients requiring more than low-flow oxygen support.- Can be performed awake
- Side-to-side positioning for patients unwilling or unable to lie prone
- Proning
Tocilizumab is indicated only for patients with evidence of systemic inflammation, defined as a CRP >75mg/L.
Supportive care:
- D
- RASS 0 to -2 in the intubated patient
- F
- Conservative fluid management
- Avoid hypervolaemia
- H
- Thromboprophylaxis
For all patients with moderate or worse disease.
- Thromboprophylaxis
Disposition:
Preventative:
- Vaccination
Marginal and Ineffective Therapies
The following are not recommended:
- Convalescent plasma
Primary driver of severe disease is inflammation, not viral load. - Hydroxychloroquine
- Famotidine
- Ivermectin
- Fluvoxamine
- Colchicine
Anaesthetic Considerations
Complications
- Death
Mortality is highly age dependent:- <10: 0.002%
- 10-25: 0.01%
- 25-55: 0.4%
- 55-65: 1.4%
- 65-75: 4.6%
- 75-85: 15%,
- >90: >25%
- B
- Pulmonary fibrosis
- Bacterial coinfection
Uncommon outside of the intubated patient.
- C
- Myocarditis
Up to 33% of critically ill patients. - Cardiomyopathy
- Myocarditis
- F
- AKI
Multifactorial:- Diuresis
- Renal microthrombi
- AKI
- H
- Venous thromboembolism
- I
- Cytokine storm
Similar to HLH.
- Cytokine storm
Prognosis
Key Studies
References
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi:10.1056/NEJMoa2001017
- Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients With Coronavirus Disease 2019 (COVID-19). Clinical Infectious Diseases. Published online September 5, 2022:ciac724. doi:10.1093/cid/ciac724
- National Clinical Evidence Taskforce. Australian guidelines for the clinical care of people with COVID-19. 2023 [version 72].
- Perkins GD, Ji C, Connolly BA, et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327(6):546-558. doi:10.1001/jama.2022.0028
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med. 2020;383(19):1813-1826. doi:10.1056/NEJMoa2007764
- Marconi VC, Ramanan AV, Bono S de, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. The Lancet Respiratory Medicine. 2021;9(12):1407-1418. doi:10.1016/S2213-2600(21)00331-3
- Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536-544. doi:10.1038/s41564-020-0695-z
- Lauer SA, Grantz KH, Bi Q, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172(9):577-582. doi:10.7326/M20-0504
- Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46(4):579-582. doi:10.1007/s00134-020-05967-x
- Guan W jie, Ni Z yi, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi:10.1056/NEJMoa2002032
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564-1567. doi:10.1056/NEJMc2004973