Deep Hypothermic Circulatory Arrest
DHCA is a neuroprotective strategy used to ↓ risk of ischaemia when surgical requirements demand complete cessation of the circulation. DHCA involves controlled cooling via CBP:
All organs are of course at risk of ischaemia during circulatory arrest, but the brain both particularly vulnerable and important.
- Cooling occurs rapidly
Generally a <10°C gradient maintained between blood and inflow temperature. - Usually to 15-18°C
Based on the anticipated duration of arrest.- Higher temperatures (22-25°C) can be used if selective cerebral perfusion is employed
- Rewarming occurs gradually
<2°C between brain and inflow temperature.
Inflow temperature is the temperature of blood returned by the CPB circuit, and is measured accurately by the machine.
Indications
- Cardiac surgery
- Aortic arch surgery
- Pulmonary thromboendarterectomy
- Complex congenital surgery
- Neurological
- Cerebral aneurysms
- Intracranial AVM
- Vena caval surgery
- Carcinoma with caval invasion
Contraindications
Principles
- Oxygen demand is a function of metabolic rate, and is strongly affected by hypothermia
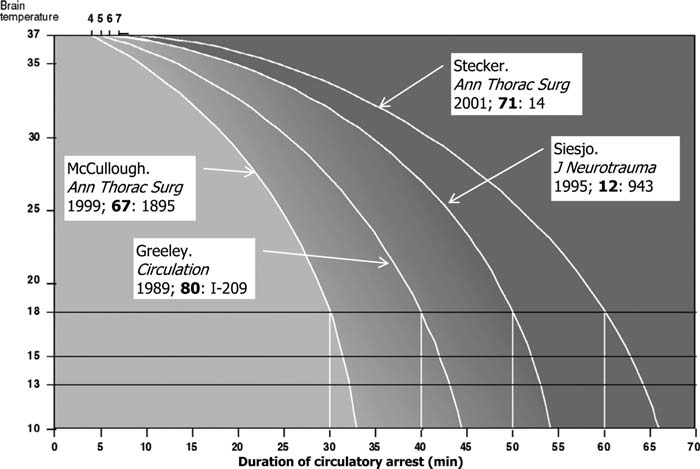
Cerebral metabolism ↓ by 6-7% for every 1°C ↓ in temperature. - Safe cerebral ischaemic time is proportional to temperature
Most patients tolerate 30 minutes of DHCA at 18°C.
Practice
Preparation
Should consider:
There is little evidence for cerebral oximetry, ice, and use of corticosteroids; most institutions will have their own policy.
There is poor correlation between rectal and pulmonary artery catheter temperature and brain temperature. Nasopharyngeal and tympanic temperature are the best surrogate.
- Cerebral oximetry monitoring
- Processed EEG monitoring
- Two temperature probes
- Brain temperature
Nasopharynx. - Body temperature
Bladder.
- Brain temperature
- Ice for topical head cooling
Delays brain rewarming during arrest, but does not contribute meaningfully to cooling. - Corticosteroids
1mg/kg prednisolone, ideally 6-8 hours before surgery.
Cooling
- Ensure adequate anticoagulation prior
- Commence topical cooling of the head
- Consider TIVA rather than volatile
Avoid uncoupling CMRO2 from metabolism. - Monitor for VF
This may result in AI and ventricular distention, which requires either:- Aortic cross-clamping and cardioplegia
- Venting
Cold
Considerations:
- Turn off all infusions, particularly vasopressors
- ↓ Metabolism of infused drugs
- No circulation of infused drugs
Will pool in the SVC, resulting in a bolus when circulation resumes.
- Acid-base measurement can use the pH or alpha-stat approach
- pH-stat may be beneficial during cooling
Adding CO2 to the CPB circuit may improve neurological outcomes through more rapid and uniform cerebral cooling.
- pH-stat may be beneficial during cooling
- Haemodilution to a haematocrit of 20% may improve microcirculatory flow
Alpha-stat vs. pH-stat is covered under Measurement Variability. In brief, alpha-stat warms a cold sample to 37°C; pH-stat adjusts the measured gas samples for the patient temperature which results in a lower partial pressure due to the ↑ in solubility.
- Adequate storage for heparinised blood
The patient is traditionally exsanguinated. - Additional return lines for cerebral perfusion
Cerebral Perfusion Strategies
Cerebral perfusion can prolong safe operating time or ↓ the degree of hypothermia required. Options include:
- Selective anterograde cerebral perfusion
Perfusion of neck vessels with 10-20mL/kg/min of blood, maintaining some cerebral blood flow.- Cannulation or grafting of one or both carotid arteries, or the axillary or brachiocephalic artery
- Right sided perfusion can be monitored targeting a right radial arterial pressure of 50-70mmHg
- NIRS is particularly helpful to note the presence of perfusion and an intact Circle of Willis, and identify any interruptions (e.g. kinking) to flow
- Intermittent systemic perfusion
Intermittent perfusion prolongs total duration of DHCA, but is not appropriate for all operations. - Retrograde cerebral perfusion
Perfusion of the brain with oxygenated blood administered retrograde into the SVC.- Relies on the fact that the cerebral vasculature has no valves
- Patchy cerebral perfusion
Retrograde cerebral perfusion likely operates mostly via the azygous system (the the IJV possess valves), whose tributaries cover (loosely) cerebral territories supplied by the posterior circulation.
| Anterograde | Retrograde | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
|
|
|
|
Rewarming
Prior to rewarming:
- De-air
- Restart infusions
During rewarming:
TEG is covered under Thromboelastography.
- Consider cold reperfusion
Restart CPB without rewarming, and continue for 10-20 minutes. - Slow rewarming to minimise tissue thermal gradients
- <2C
- Prevent secondary brain injury
Avoid:- Hyperthermia
- Hypoxaemia
- Hypotension
- Hypoperfusion
- Perform thromboelastography
Warmed sample can provide early identification of clotting factor deficiencies.
Complications
Profound hypothermia brings a raft of adverse effects:
- C
- Arrhythmia
- Proportional to degree of hypothermia
- Partially related to ↓ K+
- ↑ Plasma viscosity
- ↑ SVR
- Arrhythmia
- D
- Cerebral vasoconstriction
- ↑ Stroke risk
5-10%. Associated with:- Age
- DHCA time
- Aortic atheroma
- POCD
- Reperfusion injury
- Hyperthermic injury
- E
- Hypothermia
Temperature commonly falls after rewarming, and patients may become significantly hypothermic during the post-bypass phase.- This is ↓ by a slower rate of rewarming
- Hypothermia
- F
- ↓ GFR
- Metabolic acidosis
- ↑ BSL
↓ Glucose metabolism.
- H
- Coagulopathy
Major contributor to early and late mortality. - Thrombocytopaenia
- Coagulopathy
- Other
- Altered pharmacokinetics
Key Studies
References
- Conolly S, Arrowsmith JE, Klein AA. Deep hypothermic circulatory arrest. Continuing Education in Anaesthesia Critical Care & Pain. 2010;10(5):138-142. doi:10.1093/bjaceaccp/mkq024
- Hogue CW, Arrowsmith JE. Deep hypothermic circulatory arrest. In: Mackay JH, Arrowsmith JE, eds. Core Topics in Cardiac Anesthesia. 2nd ed. Cambridge University Press; 2012:387-394. doi:10.1017/CBO9780511979095.066