Inotropes
Inotropes are drugs that ↑ force and velocity of myocardial contraction, causing a primary ↑ SV and a secondary ↑ in CO and BP.
Inotropes can be divided based on their effect on SVR into:
- “Pure” inotropes
Predominantly naturally or synthetic catecholamines, which act to ↑ cAMP. - Inodilators
Phosphodiesterase inhibitors and calcium sensitisers.
Although we like to conceptually separate inotropes from Vasopressors, there is substantial overlap in the pharmacodynamic activity of many drugs in either category.
My preference for nomenclature is to use the umbrella term vasoactive for all agents used to manipulate haemodynamic parameters, and then categorise them based on their primary function:
- Inotrope for agents that ↑ the force of myocardial contraction
- The subcategory inodilator for drugs which also ↓ SVR
- Vasopressor for agents that ↑ SVR
- Vasodilator for agents that ↓ SVR
Principles
| Drug | Adrenaline | Dobutamine |
|---|---|---|
| Overview | Endogenous catecholamine
|
Synthetic catecholamine
|
| Dosing | 0-0.3µg/kg/min | 2.5-20µg/kg/min |
| Haemodynamics | Contractility: ↑↑ HR: ↑↑ SVR ↑↑ PVR: ↑/↓ |
Contractility: ↑ HR: ↑ SVR: ↑/↓ PVR: ↑/↓ |
| Disadvantages |
|
|
| Drug | Milrinone | Levosimendan |
|---|---|---|
| Overview | Phosphodiesterase 3 inhibitor | Calcium sensitiser that ↑ myocardial contraction with minimal ↑ in O2 demand |
| Dosing | 0-0.75µg/kg/min | 12.5mg over 24 hours |
| Haemodynamics | Contractility: ↑ HR: ↑ SVR: ↓ PVR: ↓↓ Theoretically greater effect on PVR than SVR; may be beneficial in RV disease. |
Contractility: ↑ HR: ↑ SVR: ↓ PVR: |
| Disadvantages |
|
|
Practice
- Goal is restoration of tissue perfusion
Regular clinical assessment. - Circulatory failure
- Invasive arterial monitoring
Key Studies
Cardiogenic Shock:
- DoReMi (2021)
- 192 Canadians with admitted to a single cardiac ICU in Ottawa with cardiogenic shock
- Powered to detect 20% difference in an absolutely mammoth composite primary outcome
Death, cardiac arrest, mechanical support, transplant, non-fatal MI, CVA, CRRT. - Single cardiac ICU
- Randomised with stratification based on LV, RV, or biventricular failure
- Milrinone vs. dobutamine
- Milrinone escalated in stages from 0.125-0.5μg/kg/min
- Dobutamine escalated in stages from 2.5-10μg/kg/min
- No significant difference in the composite outcome
- Similar arrhythmia and hypotension rates
- 17-24 hours between admission and randomisation may mask effects of treatment during this critical interval
Decompensated Heart Failure:
- LIDO (2002)
- 103 adult Europeans “without child-bearing potential”; with heart failure felt to be requiring an inotropic agent and have an LVEF <35%, CI <2.5, and PCWP >15mmHg
- Excluded if restrictive or hypertrophic cardiomyopathy, uncorrected stenotic valve, recent VT or VF, 2nd or 3rd degree AV block, HR >120, or other organ failure
- Multicentre (26), double-blind, double-dummy, parallel group trial
- 90% power to detect 30% ↑ in CO and 25% ↓ in PCWP at 24 hours
- Randomised to:
- Levosimendan
24μg/min over 10 minutes, followed by 0.1μg/kg/min for 24 hours. - Dobutamine
5μg/kg/min without a loading dose.
- Levosimendan
- Infusion doubled if no response within 24 hours
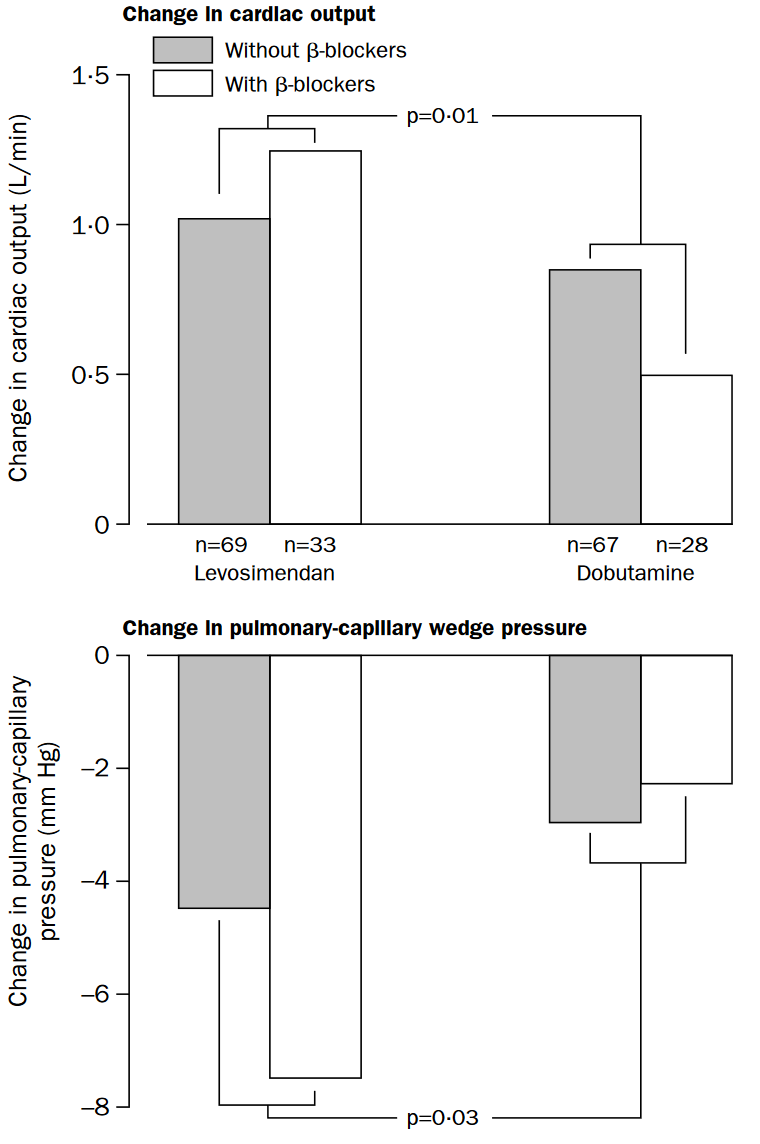
- Significantly improved haemodynamic markers in levosimendan group (28% vs. 15%, HR 1.9, p=0.02)
More pronounced in β-blocker group. - Significantly ↓ mortality in levosimendan group (8% vs. 17%)
- 103 adult Europeans “without child-bearing potential”; with heart failure felt to be requiring an inotropic agent and have an LVEF <35%, CI <2.5, and PCWP >15mmHg
- SURVIVE
- REVIVE
Cardiac Surgery:
- LEVO-CTS (2017)
- 882 adult Americans undergoing on-pump cardiac surgery with LVEF <35%, without restrictive/obstructive cardiomyopathy, renal failure, liver failure, or recent arrhythmias
- CABG, CABG + AVR, +/- MVR
- Multicentre (70), double-blind, placebo-controlled RCT
- Two primary, composite outcomes:
- Death or RRT at 30 days, MI at 5 days, or mechanical support at any time
- Death at 30 days or mechanical support at any time
- Randomised to levosimendan commenced peri-induction vs. placebo
- No change in composite outcome between groups (24.5% vs. 24.5% for 4-point outcome, 13.1% vs. 11.4% for 2-point outcome)
- 882 adult Americans undergoing on-pump cardiac surgery with LVEF <35%, without restrictive/obstructive cardiomyopathy, renal failure, liver failure, or recent arrhythmias
- CHEETAH (2017)
- 506 adults undergoing cardiac surgery with severe cardiovascular dysfunction (LVEF <25%, pre-operative IABP, intraoperative IABP required)
- Multicentre (14) parallel-group trial across Italy, Russia, and Brazil
- 1000 patients provides 80% power for 5% ↓ in absolute mortality
- Levosimendan at 0.05-0.2μg/kg/min for up to 48 hours vs. placebo
- Terminated early for futility
- No difference in mortality
Sepsis:
- LeoPARDS (2017)
- 516 British adults with suspected sepsis ( by possible infection and ⩾2 SIRS criteria)
- Multicentre (34), double-blind, placebo-controlled RCT
- 90% power to detect a mean difference in SOFA score of 0.5
- Levosimendan vs. standard care
- Levosimendan
0.1µg/kg/min, ↑ to 0.2µg/kg/min at 2-4 hours if tolerated and continued for up to 24 hours. - Placebo
- Levosimendan
- Study drug commenced after adequate fluid resuscitation and restoration of target MAP
- No difference in mean SOFA score, ↑ haemodynamic instability and mean ventilator days in levosimendan group
References
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895–1032.
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Mathew R, Di Santo P, Jung RG, et al. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. New England Journal of Medicine. 2021;385(6):516-525.