Intracranial Pressure
Indications
Contraindications
Anatomy
Pathophysiology
Normal ICP waveform contains 3 peaks:
- P1
Percussion wave from arterial pressure transmitted to the choroid plexus. - P2
Tidal wave due to brain compliance. P2 > P1 when brain compliance falls. - P3
Dicrotic wave from AV closure.
Lundberg waves are pathological waveforms seen rarely:
- Lundberg A waves
Slow rise in ICP to very high (50-100mmHg) levels, occurring due to a fall in MAP/CPP leading to cerebral vasodilatation and ↑ ICP. Can be terminated by an ↑ in MAP and restoration of CPP. - Lundberg B waves
Changes in ICP by 10-20mmHg over shorter (30-180s) time periods, with associated change in respiratory pattern. - Lundberg C waves
Low amplitude changes in ICP that occur several times per minute.
Clinical Manifestations
Clinical features of ↑ ICP include:
- Headache
- Nausea/vomiting
- Hypertension
With associated ↓ HR. - Progressive loss of consciousness
- GCS ⩽12 is relative contraindication to LP without radiographic exclusion of ↑ ICP or hydrocephalus
- Coma is a late sign in isolated ↑ ICP
- Focal neurological signs
- Seizures
- Papilloedema
- Unilateral pupillary dilation with loss of reactivity
- Suggests transtentorial herniation
- Typically followed by loss of brainstem and cranial nerve function
In absence of intracranial injury and preserved cerebral autoregulation, ICP may rise as high as 60mmHg before consciousness is impaired.
Monitoring
ICP monitoring is indicated:
- For salvageable patients with GCS ⩽8 and TBI, after resuscitation with:
- Abnormal CT scan
Swelling, ICH, hydrocephalus, herniation. - Normal CT scan but ⩾2 of:
- Age >40
- Motor posturing
- SBP <90mmHg
- Abnormal CT scan
- Until ICP has stabilised and clinical assessment can be performed
Commonly used systems for measuring ICP include:
- Intraparenchymal transducer
- Intraventricular catheter
- Multimodality monitoring
Intraparenchymal Transducer
Intraparenchymal transducers:
- Assess regional pressure
- Can be inserted at the bedside via small burr hole
Ideally done in OR for sterility. - Include:
- Fibre-optic
Camino. - Strain-gauge
Codman.
- Fibre-optic
- Accuracy drifts over time
Intraventricular Catheter
Intraventricular catheters (EVD):
- Provide an assessment of global ICP
- Facilitate drainage and sampling of CSF
- More difficult to place with severe brain oedema and ↓ ventricle size
- Can be regularly re-calibrated
Remain accurate over time.
Multimodality Monitoring
A variety of other intracranial monitors see use in specialist centres, although without much evidence supporting them. These include:
- Intraparenchymal oxygen content
Invasive measurement of cerebral oxygen tension.- Normal 20-35mmHg
- <10mmHg associated with ↑ morbidity and mortality
- <20mmHg suggested as threshold for intervention
- Jugular bulb oxygen saturation
Invasive measurement of cerebral venous oxygen saturation.- Normal 55-70%
- Low levels suggested oxygen supply-demand mismatch
- Elevated levels suggest hyperaemia or brain death
- Cerebral Microdialysis
Invasive measurement of cerebral interstitial metabolic milieu via microdialysis probes.- Measurement of:
- Lactate
- Pyruvate
- Glucose
- Glutamate
- Glycerol
- Lactate and pyruvate used as markers of ischaemia
- Measurement of:
- Transcranial Doppler
Noninvasive measurement of cerebral blood flow. - NIRS
Non-invasive measurement of cerebral oxygen saturation. Not validated in neurological intensive care.
Management
Broadly divided into three tiers:
Although evidence exists for these therapies in isolation, there is little evidence demonstrating effect of any combination or process. However, they are commonly arranged into tiers based on invasiveness and side-effect profile to provide a framework treatment and escalation.
- Tier 1
- Sedation and analgesia to ↓ ICP
- Benzodiazepine
- Opioid
- Maintain CPP 60-70mmHg
- Fluid boluses, avoiding hypervolaemia
- Vasopressors
- Low-normal CO2
Tight control aiming PaCO2 of 35-38mmHg. - Osmotherapy ↓ cerebral oedema:
- Mannitol 0.25-1g/kg
- Creates an osmolar gap
- Enters brain via damaged BBB, which may result in local ↑ ICP
- Hypertonic saline
3mmol ↑ in osmolality ↓ cerebral volume by ~7%.- 5-10mL 23.4%
- Mannitol 0.25-1g/kg
- Drain CSF
- Consider EVD if only parenchymal probe is placed
- Set at 10mmHg with EVD
- Should be vented continuously, not intermittently
- Consider seizure prophylaxis
- Sedation and analgesia to ↓ ICP
There is no evidence supporting mannitol or hypertonic saline over the other, however I prefer hypertonic saline due to:
- Repeatability
- Preservation of osmolar gap
- Tier 2:
- Neuromuscular blockade
Trial paralysis - if effective, produce with continuous infusion.- Remove hard cervical spine collar
If present.
- Remove hard cervical spine collar
- Mild ↓ CO2
Tight control aiming PaCO2 of 32-35mmHg. Caution should be used if brain tissue oxygenation mointoring is not available. - Consider MAP challenge
- Neuromuscular blockade
At each stage, reconsider the:
- Need for surgical intervention for potentially correctable lesions
- Effect of extracranial causes for ↑ ICP
- Tier 3:
- Thiopentone coma
- Profound ↓ CMRO2
- Long half-life
Delays further neuro-prognostication. - EEG monitoring recommended to avoid titrating past burst suppression
- Secondary decompressive craniectomy
- Aggressive hyperventilation
- Hyperventilation to hypocarbia does ↓ CO2 and ↓ ICP, but leads to cerebral vasoconstriction and ↓ CBF
- Has a role for short term control of ↑ ICP prior to some definitive therapy, or in response to tonsilar herniation
e.g. the patient who blows a pupil during transfer to the OR. - Should be avoided during first 24 hours of a TBI, as CBF is critically ↓ over this period
- Thiopentone coma
MAP Challenge
Principles:
- Tests whether patients intrinsic CPP requirement is higher than the standard 60-70mmHg
- Success requires both:
- Intact cerebral static pressure autoregulation
- Current CPP < lower breakpoint of static pressure autoregulation
- Cerebral autoregulation may not be stable and so regular revaluation is required
Practice:
- Pharmacologically ↑ MAP by 10mmHg for up to 20 minutes
- Observe the change in:
- MAP
- ICP
- CPP
- PbtO2
- A successful MAP challenge:
- Results in a ↓ ICP for an ↑ CPP and MAP
- Occurs because intact autoregulation causes vasoconstriction in response to ↑ CPP, and therefore ↓ cerebral blood volume
- An unsuccessful MAP challenge:
- Results in ↑ ICP as cerebral blood volume ↑ in absence of functioning autoregulation
- Adjust blood pressure targets accordingly
Complications
Key Studies
Decompressive Craniectomy:
- DECRA (2011)
- 155 adults with severe TBI within 72 hours of injury:
- With uncontrolled ICP (>20mmHg for 15 minutes)
- For full active treatment
- Without spinal cord injury or intracranial mass lesions
- Bifrontotemporoparietal craniectomy with bilateral dural opening vs standard care
- Control group could receive “lifesaving” craniectomy at 72 hours
- Standard ICP treatment in both groups
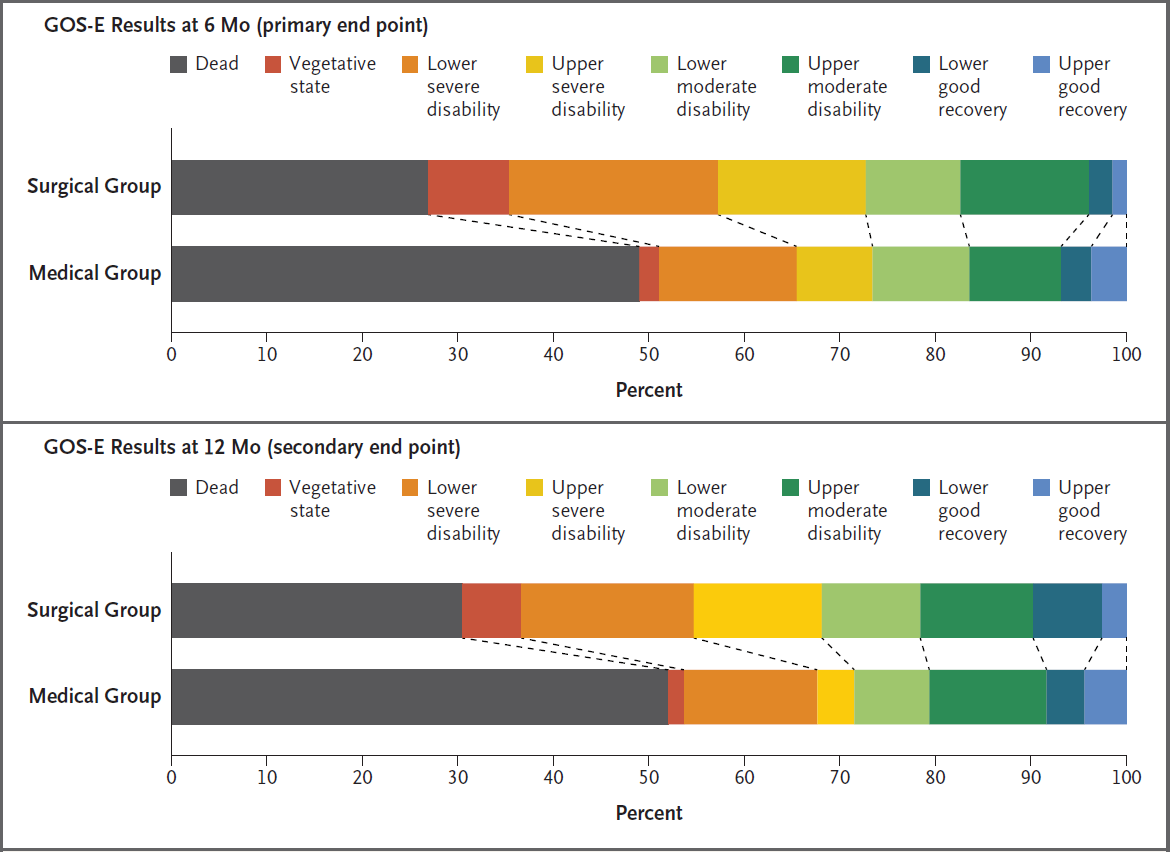
- Significant ↑ (70% vs 51%) in unfavourable (GOS-E 1-4) outcomes at 6 months
- Significant ↓ in ICP in intervention group
- 25% of control group crossed over
- 155 adults with severe TBI within 72 hours of injury:
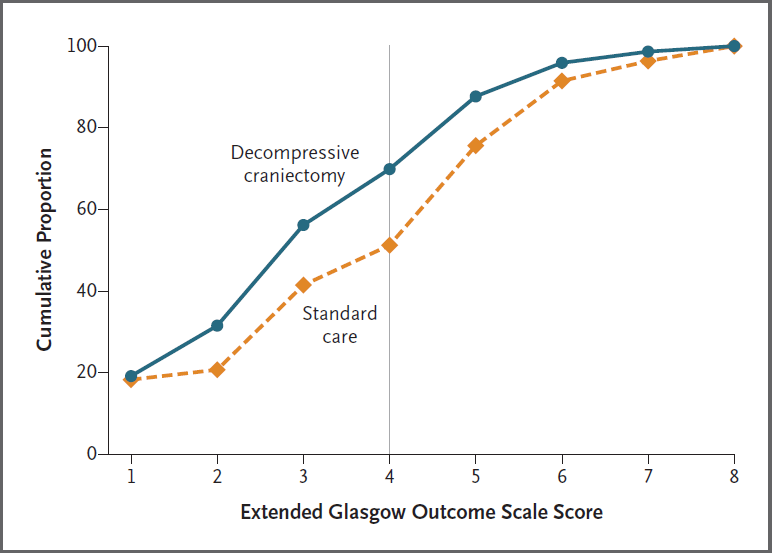
- RESCUE ICP (2016)
- 408 patients aged 10-65 with TBI, abnormal CT, and ICP >25mmHg for 1-12 hours, despite tier 1 and 2 therapies
- Multicentre (52), international, parallel group, block mutated RCT, statified by site
- 400 patients gave 80% for 15% difference in favourable neurological outcome
- Decompressive craniectomy vs. standard care
- Decompressive craniectomy
- Large unilateral frontotemporoparietal craniectomy for unilateral swelling
- Bifrontal craniectomy for diffuse swelling
- Medical care
- Barbiturates
- Crossover to craniectomy permitted if decline, which occurred in 37% of patients
- Standard ICP control measures in both groups
- Decompressive craniectomy
- Decompressive craniectomy resulted in ↑ survival with a bad neurological outcome
- Slow recruitment
- High crossover from medical group
- Investigators estimated for every 100 patients receiving decompressive craniectomy there were:
- 22 survivors
- 6 vegetative
- 8 lower-severe disability
- 8 upper-severe disability
- 22 survivors
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Hawryluk GWJ, Rubiano AM, Totten AM, et al. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery. 2020;87(3):427-434.
- Carney N, Totten AM, OʼReilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition: Neurosurgery. Published online September 2016:1.
- Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019;45(12):1783-1794. doi:10.1007/s00134-019-05805-9
- Hutchinson PJ, Kolias AG, Timofeev IS, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N Engl J Med. 2016;375(12):1119-1130. doi:10.1056/NEJMoa1605215
- Dünser MW, Dankl D, Petros S, Mer M. Clinical Examination Skills in the Adult Critically Ill Patient. Springer International Publishing; 2018.