Principles of Antimicrobial Use
Key principles of antimicrobial use include:
Empirical antibiotics are those given to cover likely organisms, rather than given in response to a known pathogen.
Inappropriate or delayed antibiotic use ↑ morbidity and mortality.
- Sensible prescribing
- Appropriate sourcing and prescription of cultures
- Antimicrobial characteristics
- Minimum inhibitory concentration
- Kill characteristics
- Post-antibiotic effect
- Treatment failure
- Dose adjustment
Prescribing
Principles:
- Antibiotics should be initiated for:
- Shock likely related to infection
- High likelihood of bacterial infection
- Patient condition does not allow watchful-waiting
- Antibiotics should not be started if:
- Shock not related to infection
- Viral infection
- Source control alone would be sufficient
- Patient condition allows watchful-waiting
- Administer antibiotics without delay
- Choice of empirical antimicrobial should be based upon:
- Site of infection
- Community or hospital-acquired
- Local microbiome
Understanding of the hospital or intensive care units antibiogram. - Patients previous microbiology results
- The narrowest-spectrum drug should be used
Ideally empirical spectrum can be narrowed after 3 days. - Drugs eliminate susceptible organisms, at the cost of significant side-effects
- Provide huge evolutionary advantage to resistant strains
- Promote overgrowth of non-susceptible organisms
- Including fungi
- Monotherapy is preferable to combination therapy
Exceptions include:- Achieving adequate empiric cover
- Treatment of polymicrobial infections with known sensitivities
- Antibiotic synergy
- Neutropaenic sepsis
- Known bacteraemia
- High (>25%) mortality
- Clinical response should be used to guide therapy
- In vitro sensitivity does not equate with clinical effectiveness
- In vitro resistance is a better predictor than sensitivity
- A >7 day course is rarely indicated
- Prolonged courses are associated with significant adverse effects
- Exceptions include:
- Immunosuppressed patients
- Osteomyelitis
- Infective endocarditis
- Complicated bacteraemia
Require longer therapy and have ↑ mortality. Indicated by:- CNS involvement
- Septic emboli
- Multiple infected regions
- Recurrent infection
- An adequate dose should be given
Under-dosing leads to resistance. - Serum levels of toxic antibiotics should be monitored for both toxicity and efficacy
- Recognise patients with augmented renal clearance, and ↑ dose accordingly
| Category | Factor | Examples |
|---|---|---|
| Patient | Age |
|
| Allergies |
|
|
| Metabolic function |
|
|
| Pregnancy |
|
|
| Immunocompetence |
|
|
| Genetic |
|
|
| Epidemiological | Travel |
|
| Occupational |
|
|
| Recreational |
|
|
| Therapeutic |
|
|
| Disease | Clinical |
|
| Infection |
|
|
| Organism |
|
|
| Drug | Pragmatic |
|
| Pharmacokinetics |
|
|
| Pharmacodynamic |
|
Microbial Cultures
- Microbiological specimens should be obtained before commencing antimicrobials
- Prevents false-negative cultures
- Immediate Gram stain may guide therapy
- Interpretation of cultures depends on the site:
- Sterile sites:
- Include blood, urine, CSF
- Cultured organisms are significant and indicate infection
- Usually only one organism cultured, which is the causative organism
- Non-sterile sites:
- Usual commensal organisms are expected
- Diagnosis of infection is clinical
Exception is cultured organisms that are definitively not commensal:- Legionella
- TB
- Microbiological results guide treatment
- Sterile sites:
- Gram negative organisms become dominant with hospital admission
- The hallmark of an intravascular device infection (including CLABSI) is a continuous bacteraemia
True bacteraemia (or fungaemia) should be assumed with culture of:
- S. Aureus
- E. Coli
- Candida
An antibiogram is a summary of the local antimicrobial susceptibilities of different pathogens, and is important to determine choices for antimicrobial agent for both empirical therapy, and prior to receiving sensitivity data.
Drug Properties
Minimum Inhibitory Concentration
The MIC is the lowest concentration of antimicrobial that will inhibit visible growth of an organism after overnight incubation. The MIC is:
- Reported as μg/mL
A lower MIC indicates ↑ efficacy. - Easily performed
Usually automated, and therefore reproducible - Affected significantly by:
- Incubation time
↑ MIC with ↑ time. - Concentration of bacteria in sample
↓ MIC with ↓ concentration.
- Incubation time
- Not a perfect surrogate for in vivo efficacy
- Other factors besides MIC determine antibiotic effect
- Antibiotic tissue concentration (at site of disease) may be higher or lower than sampled serum concentration
Kill Characteristics
Antibiotics can effect bacterial genocide in three broad ways:
- Time-dependent killing
Efficacy dependent on the time that antibiotic concentration exceeds MIC, but not by how much.- Occurs when the drug only works at a particular point in the bacterial life cycle
e.g. Preparation for cell division. - Threshold effect
- Maximal effect reached at some proportion (usually 40-70%) of the dosing interval
Drug concentrations do not have to be continuously over MIC. - Affected by tissue penetration
Time over MIC is important only in the tissue where the infection is, which may not be in blood (where we measure concentrations).
- Maximal effect reached at some proportion (usually 40-70%) of the dosing interval
- Lend themselves to dosing by infusion
- Benefits (at steady-state) include:
- ↓ Mortality compared to intermittent dosing
- ↑ Time above MIC, which should ↑ efficacy and ↓
- ↓ Peak concentration, which generally ↓ toxicity
- ↓ Nursing workload
- Disadvantages include:
- ↓ Concentration gradient driving diffusion into tissues
- Most drugs are not pure time-dependent killers, and ↑ concentration still results in some ↑ efficacy
- MIC is not known in early stages of treatment
- Requires continuous use of a lumen
- May ↓ ventilator days without clear evidence of mortality benefit, with greatest effect seen in the critically ill
- Benefits (at steady-state) include:
- Examples include:
- β-lactam
- Carbapenems
- Linezolid
- Occurs when the drug only works at a particular point in the bacterial life cycle
- Concentration-dependent killing
Efficacy dependent on how much antibiotic concentration exceeds MIC, but not by how long for.- Occurs when drug affects some critical component of bacterial infrastructure
- Greater effect with greater dose
- Examples include:
- Aminoglycosides
- Metronidazole
- Fluoroquinolones
- Occurs when drug affects some critical component of bacterial infrastructure
- Time and concentration-dependent killing
Efficacy dependent on both time over MIC and concentration above MIC.- Occurs for drugs which prevent synthesis of components for cell division
- ↑ Concentration results in ↑ inhibition of relevant components
- ↑ Time results in affecting a greater number of bacteria
As they won’t all divide at the same time.
- Examples include:
- Fluoroquinolones
- Azithromycin
- Glycopeptides
- Occurs for drugs which prevent synthesis of components for cell division
Post-Antibiotic Effect
The post-antibiotic effect describes bacterial killing that persists after the concentration falls below MIC. Post-antibiotic effects:
- Are an idiosyncratic feature of some agents
Usually where the drug binds strongly to some part of the bacteria. - Are strongest generally for drugs with concentration-dependent killing
Treatment Failure
Antibiotic treatment failure may occur due to:
- Wrong bug
- Not susceptible
- Viral
- Fungal
- Parasitic
- Not infection
- Not susceptible
- Wrong drug
- Resistant organism
- Poor penetration to infected tissue
- Bacteriostatic antagonism
Administration of a bacteriostatic antibiotic may ↓ efficacy of a bacteriocidal antibiotic, as cell division cannot be interrupted if it does not occur.
- Wrong dose
- Too small
- Too infrequent
- Too short
- Wrong route
- Inadequate oral absorption
- Source control
- Incomplete surgical resection
- Inadequate sputum clearance
Dose Adjustment
| Characteristic | Increased With | Decreased With |
|---|---|---|
| Peak Target Concentration |
|
|
| Half-life |
|
|
Renal Failure
Adjusting antibiotic administration in renal failure is to avoid toxicity as efficacy is unaffected by impaired renal clearance. Drugs may require:
In general:
- Interval-adjustment:
- Aminoglycosides
- Fluoroquinolones
- Glycopeptides
- Dose or Interval adjustment:
For drugs not particularly toxic in overdose either can be chosen, although interval adjustment is cheaper.- Beta-lactams
- Carbapenems
- Dose adjusted
- Best for both:
- Time-dependent killing
Time above MIC determines efficacy, but amount above MIC doesn’t. - Concentration-dependent toxicity
- Time-dependent killing
- Maintains time above MIC, with ↓ dosing to avoid progressively ↑ drug levels when amount given exceeds amount cleared
- Best for both:
- Interval adjusted
- Best for both:
- Concentration-dependent killing
Peak concentration should be 8-10 times MIC, but the time above MIC doesn’t effect efficacy. - Concentration-dependent toxicity
- Concentration-dependent killing
- Maintains the high peak concentration required for killing, but minimises concentration at other times
- Best for both:
Renal Replacement Therapy
Dose adjustment on RRT is similar to renal failure. In general, drugs can require:
- No adjustment
Administration is unchanged due to:- Small free drug fraction
- High VD
- Highly protein bound
- Hepatically cleared
- Cleared by filtration
CRRT replicates glomerular filtration reasonably well, but not other forms of renal clearance.
- Small free drug fraction
- Interval adjustment
- Actively transported drugs
Renal clearance by tubular secretion is not replicated by CRRT, and so interval adjustment needed to ↓ toxicity.
- Actively transported drugs
- Dose adjustment
- Renally reabsorbed drugs
Drugs that are usually reabsorbed by the tubule undergo increased clearance in the kidney, and therefore require ↑ dose on RRT.
- Renally reabsorbed drugs
Key Studies
- FABLED (2019)
- Adults in the ED with suspected severe sepsis
- Multi-centre (7) diagnostic cohort study
- 2 sets of blood cultures taken before antibiotics via separate venepuncture
- 1-2 sets of cultures taken 30-240 minutes after antibiotics
- Significant ↓ in positive cultures post antibiotics
- ~30% positive pre-antibiotic, compared to ~20% positive post-antibiotic
RRR ~33%. - More pronounced ↓ in positive post-antibiotic cultures if organism was sensitive to antibiotic used
RRR ~50%. - Positive post-antibiotic cultures were associated with ↑ time to positivity, suggesting ↑ bacterial burden
- ~30% positive pre-antibiotic, compared to ~20% positive post-antibiotic
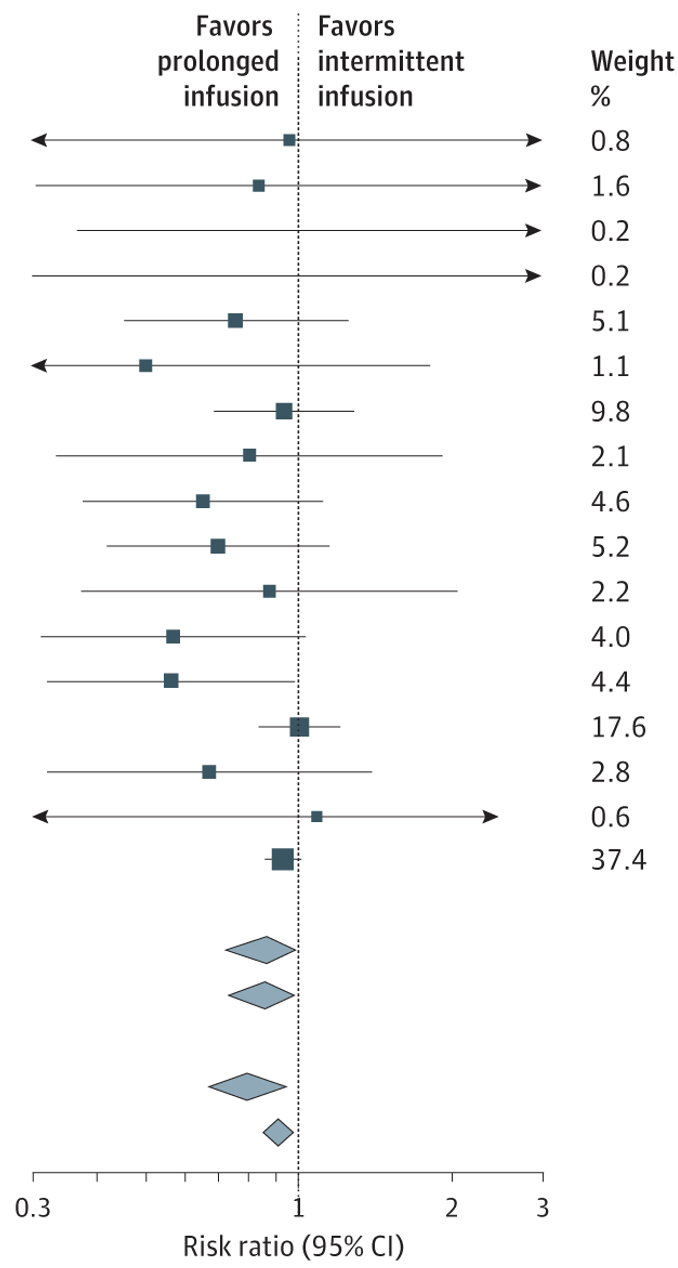
- Prolonged vs Intermittent Infusions of β-Lactam Antibiotics in Adults With Sepsis or Septic Shock (2024)
- β-Lactam antibiotics are commonly used as first-line agents in sepsis, and their bactericidal activity is related to time-above MIC
- A series of RCTs comparing intermittent dosing to continuous infusion have been performed
These include BLING-II, MERCY, and BLING-III. - Meta-analysis was strongly suggestive of ↓ 90-day mortality with the use of an intermittent infusion
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Cheng MP, Stenstrom R, Paquette K, et al. Blood Culture Results Before and After Antimicrobial Administration in Patients With Severe Manifestations of Sepsis. Ann Intern Med. 2019;171(8):547-554. doi:10.7326/M19-1696
- Timsit JF, Ling L, De Montmollin E, Bracht H, Conway-Morris A, De Bus L, et al. Antibiotic therapy for severe bacterial infections. Intensive Care Med [Internet]. 2025 Sept 1.