Delirium
Acute confusional state secondary to an organic brain disorder that is:
- Fluctuant
- Characterised by impairments in:
- Orientation
To time, place, and person. - Attention
- Grasp
Lack of contextual understanding - does not appreciate they are a patient, you are a clinician, they are in a hospital.
- Orientation
- Divided into three subtypes:
- Hypoactive
Sedated and inattentive, but docile and cooperative meaning that it is relatively underdiagnosed. - Hyperactive
Agitated, aggressive, and uncooperative. - Mixed
Fluctuant between hypoactive and hyperactive forms.
- Hypoactive
Delirium tremens is a subtype of delirium occurring 48-72 hours after alcohol withdrawal, and is covered elsewhere.
Epidemiology and Risk Factors
Delirium is exceedingly common in inpatients:
- Occurring in:
- ~70% of ventilated ICU patients
- ~50% of surgical patients
- ~30% of medical patients
- Majority are hypoactive delirium
Despite this documented high prevalence, it is likely delirium is still under-diagnosed.
| Modifiable | Non-Modifiable |
|---|---|
|
|
Pathophysiology
Poorly understood; proposed mechanisms include:
- TNF-α activation in microglia
- Altered cerebral blood flow
- Thalamic dysfunction
- Relative dopamine excess
Aetiology
Clinical Manifestations
Diagnostic Approach and DDx
Screening for delirium is recommended:
- By major intensive care societies
- Regularly
- Usually daily in appropriate patients.
- Unclear how this should best be performed in practice
- Sensitivity and specificity of screening tools varies widely between publications
Likely affected substantially by implementation and local practice.
- Using standardised tools
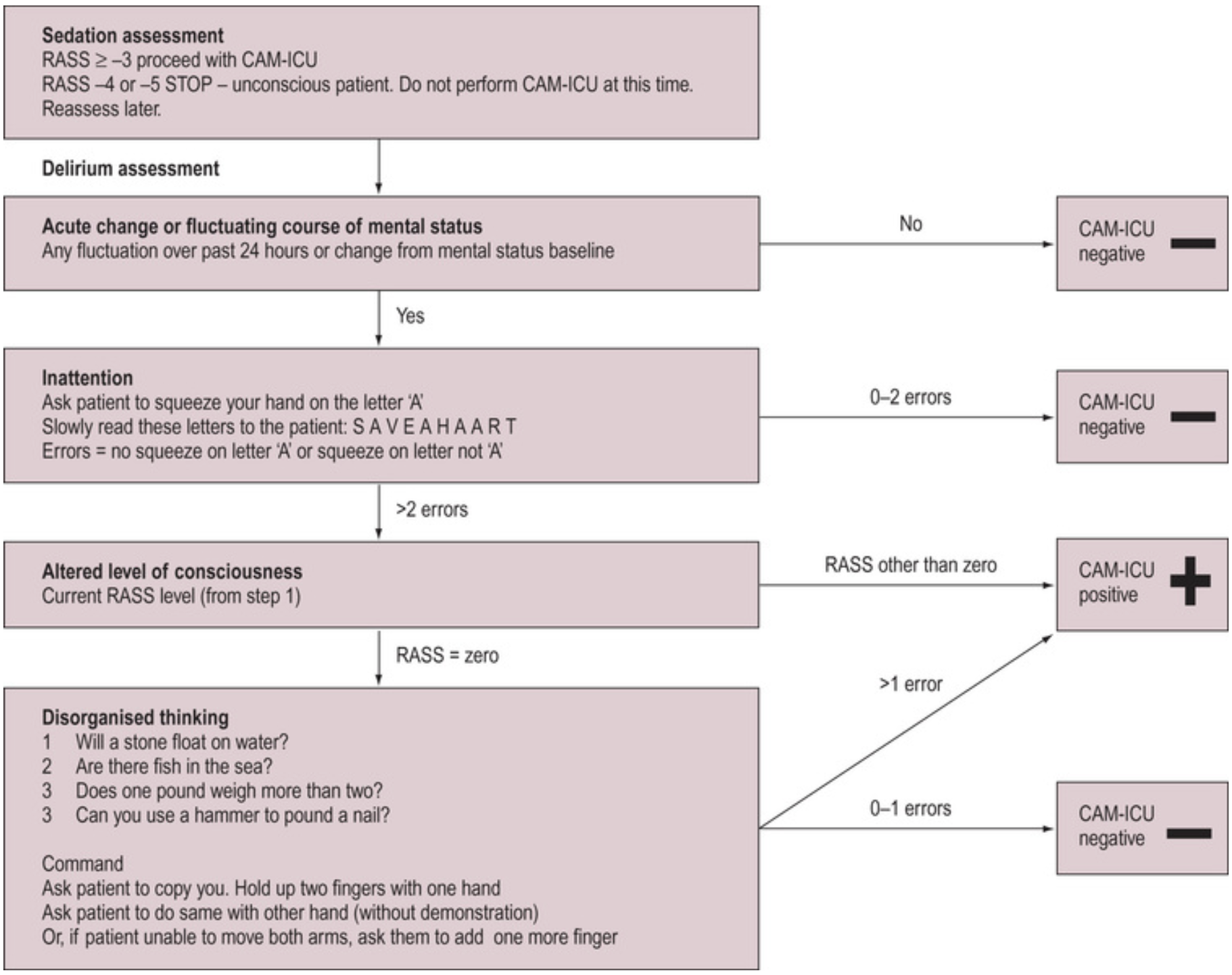
- Confusion Assessment Model (CAM-ICU)
- Intensive Care Delirium Screening Checklist (ICDSC)
Both scores are designed for binary prediction (present/absent) of delirium, although quantification of a spectrum of delirium is also possible.
Confusion Assessment Model
The CAM-ICU score is:
- A point in time assessment
- Specific but not sensitive for delirium
- Relatively quick and straightforward to perform
- Requires prior calculation of the RASS
The RASS is a 10-point scale that grades patient arousal from:
- +4: Combative
Violent, with immediate danger to staff. - +3: Very agitated
Pulls or removes indwelling devices. - +2: Agitated
Non-purposeful, fights ventilator. - +1: Restless
Anxious but not aggressive. - 0: Alert and calm
- -1: Drowsy
Sustained (>10s) awakening to voice. - -2: Light sedation
Brief awakening to voice. - -3: Moderate sedation
Movement or eye opening to voice. - -4: Deep sedation
Movement or eye opening to pain. - -5: Unarousable
No response to pain.
Investigations
Bedside:
Laboratory:
Imaging:
Other:
Management
- Maximise preventative interventions
Predominantly non-pharmacological. - Correct cause
- Minimise pharmacological intervention
- Remember the family
Emphasise delirium is common and transient.
Specific therapy:
- Pharmacological
- Antipsychotics
- Typical antipsychotics
- Haloperidol
- 0.5-10mg, usually 1-2mg IV
- Risk of QTc prolongation
- Haloperidol
- Atypical antipsychotics
Lower extrapyramidal side effects.- Olanzapine
- 2.5-5mg PO BD
- Can be given via sublingual wafers
- Quetiapine
- 12.5-50mg PO BD
- Risperidone
- 0.5-1mg PO BD
- Olanzapine
- Typical antipsychotics
- α2-agonists
- Clonidine
- Dexmedetomidine
- Antipsychotics
- Physical
Non-pharmacological interventions are covered under preventative management, but remain a critical part of treating active delirium.
Extrapyramidal side effects are adverse drug-induced movement disorders secondary to dopamine-receptor antagonism, and include:
- Dystonia
Involuntary muscle contraction, with abnormal posturing and repetitive movements.- Oculogyric crisis
Bilateral upward gaze. - Opisthotonus
Severe spine/neck hyperextension. - Torticollis
- Trismus
- Oculogyric crisis
- Akathisia
Physical restlessness. - Tardive dyskinesia
Tongue and facial choreoathetoid movements, which may impair swallowing. - Parkinsonism
- Pharyngeal dystonia
May precipitate airway crises.
Supportive care:
Disposition:
Preventative:
- Orientation aids
- Orientation clues
- Windows with natural light
- Clocks
- Calendars with todays date
- Verbal statements of the time, date, and location
- Familiar individuals
- Family
- Greatest familiarity
- Sense of control
- Sense of safety
- Nursing and medical staff
- Family
- Orientation clues
- Minimise disorientation
- Sensory impairments
- Hearing aids
- Glasses
- Sensory impairments
- Minimise agitation
- Environmental noise
- Sleep quality
- Physical restraints
- Medical restraints
- IDC
- IV lines
- Address risk factors
- Minimise sedation
Avoid deep sedation without good reason. - Pain
- Bowel care
- Polypharmacy
- Appropriate oxygenation and haemodynamic target
- Electrolyte correction
- Minimise sedation
- Patient diaries
- Fill gaps in memory after ICU discharge
- Rationalise traumatic hallucinations
Balancing opioid related harm and pain management is a difficult balancing act. On balance, I am to ensure adequate analgesia (minimising opioid when able, and using lower-risk opioids) and accept some opioid related harm.
Marginal and Ineffective Therapies
- Benzodiazepines
Significant independent risk factor for delirium, and recommended only for:- Delirium tremens
- Urgent control of dangerous hyperactive delirium
- Prophylactic antipsychotics
May ↓ duration or severity, but no improvement in mortality. - Anticholinesterases
Worsen delirium. - Melatonin
No change in rate of delirium, or quality and quantity of sleep.
Anaesthetic Considerations
Complications
Prognosis
Key Studies
- Pro-MEDIC (2022)
- ~850 Australian adults across 12 ICUs with expected ICU admission >72 hours
- 4mg enteric melatonin nocte for 14 days vs. placebo
- No change in delirium (by CAM-ICU) or sleep quality or quantity
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Krewulak, K.D., Rosgen, B.K., Ely, E.W., Stelfox, H.T., Fiest, K.M., 2020. The CAM-ICU-7 and ICDSC as measures of delirium severity in critically ill adult patients. PLoS One 15, e0242378.
- Wibrow B, Martinez FE, Myers E, et al. Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): a randomized controlled trial. Intensive Care Med. 2022;48(4):414-425. doi:10.1007/s00134-022-06638-9