Acute Respiratory Distress Syndrome
Bilateral diffuse inflammatory lung injury characterised by loss of aerated lung tissue and reduced pulmonary compliance. Various diagnostic criteria exist, the Berlin Criteria requires all of:
The Berlin criteria were updated in 2023 to remove the reliance on ABG and radiography, and include the use of HFNO. Whilst this makes the definition more widely available, it also weakens discrimination of an already over-broad definition and so further limits the utility of the concept of ARDS.
- Acute onset
Within 7 days of known precipitant. - Bilateral radiographic opacities
Consistent with pulmonary oedema. Can be on:- XR
- CT
- Ultrasound
With a well-trained operator
- Non-hydrostatic
Not related to an ↑ PCWP. If access to the following equipment is available, then it is present despite:- Intubation
- NIV with CPAP ⩾5cmH2O
- HFNO ⩾30L/min
- Hypoxia
ARDS is graded by severity of hypoxia into:- Mild
- PaO2/FiO2: 200-300
- SpO2/FiO2 <315 with SpO2 <97%
- Moderate
PaO2/FiO2: 100-200. - Severe
PaO2/FiO2: <100.
- Mild
The PaO2:FiO2 (P:F) and SpO2:FiO2 and (S:F) ratios quantify the degree of hypoxia relative to the degree of oxygen supplementation.
They are calculated as:
- \(P/F = {PaO_2 \over FiO_2}\)
- \(S/F = {SpO_2 \over FiO_2} \times 1000\)
Where:
- \(PaO_2\) is in mmHg
- \(FiO_2\) is a decimal (0-1)
Epidemiology and Risk Factors
Significant regional variation, in part due to different definitions. In general, ARDS:
- Represents ~10% of ICU admissions
- ~25% of those requiring mechanical ventilation
- Cases are reasonably spread across all severities:
- ~30% are mild
- ~45% are moderate
- ~25% are severe
Pathophysiology
Acute phase consists of a combination of:
- Alveolar-capillary barrier damage
Bidirectional leakage of fluid and protein:- Plasma protein into alveolus
Causing pulmonary oedema. - Alveolar protein into plasma
Surfactant and other alveolar proteins.
- Plasma protein into alveolus
- Inflammatory infiltrate
Large ↑ in:- Alveolar macrophages
Usually dominant cell in health. - Alveolar neutrophils
Rare in health (~1% of cells), become superabundant (~90%) in ARDS.
- Alveolar macrophages
- Surfactant dysfunction
Epithelial cell damage alters cellular production of inflammatory mediators and ↑ water egress into the alveolus, as well as production of dysfunctional surfactant.
The late (>5 days) phase is characterised by fibrosing alveolitis.
Aetiology
| Direct | Indirect |
|---|---|
| Pneumonia | Sepsis |
| Aspiration | Trauma |
| Contusion | Massive transfusion |
| Fat embolism | Pancreatitis |
| Drowning | CPB |
| Inhalational injury | |
| Reperfusion injury |
Clinical Manifestations
Diagnostic Approach and DDx
Investigations
Management
Principles of management are:
- Early identification and treatment of cause
- Avoidance of further lung injury
Specific therapy:
- Pharmacological
- Steroids
Likely ↓ mortality and ventilator duration.- Indications depend on cause, and include:
- Primarily infective
- Steroid-responsive
Organising pneumonias. - Moderate-severe ARDS that fails standard therapies
- Consider:
- Dexamethasone 10mg IV OD
- Methylprednisolone
- Pure glucocorticoid preferable to mineralocorticoid
Avoids sodium and water retention.
- Indications depend on cause, and include:
- Steroids
- Physical
- Proning
Significant improvement in hypoxia by:- Recruiting dorsal lung
- Improving V/Q matching
- Proning
| Time | Dose |
|---|---|
| Load | 1 mg/kg |
| Day 1-14 | 1 mg/kg/day |
| Day 15-21 | 0.5 mg/kg/day |
| Day 22-25 | 0.25 mg/kg/day |
| Day 26-28 | 0.125 mg/kg/day |
| Note: If the patient extubates during day 1-14, advance to day 15 of the schedule | |
Proning is a cornerstone of ARDS management, and is covered in detail under Proning.
Supportive care:
- B
- Lung protective ventilation
- Appropriate PEEP
Often titrated to a PEEP-FiO2 table. Considerations:- The disease and degree of recruitable lung is highly heterogenous and often over-distension and under-recruitment will occur together
- Higher PEEP is associated with better outcomes in more severe disease, and worse outcomes in milder disease
- Inadequate PEEP tends to cause under-recruitment and hypoxia
- Excessive PEEP tends to cause over-distension, ↑ lung stress, and reduced cardiac output
- Ideally, PEEP maintains lung volume at FRC thereby:
- Maximally recruiting available alveoli
- Spreading VT over a greater number of alveoli
- Reducing Pip
- Deceasing Barotrauma and volutrauma
- Appropriate FiO2
High FiO2 is associated with diffuse alveolar damage. FiO2 should be titrated (in combination with PEEP adjustment):- Down to <0.6 (as able)
- Aiming SpO2 >90%
- Permissive hypercapnoea
- Avoid:
- Volutrauma
AimVT4-6mL/kg predicted body weight. - Barotrauma
Aim Pplat <30cmH2O.- The plateau pressure is a substitute for the transpulmonary pressure, which reflects the actual elastic distending pressure of the lung
- Measurement of the transpulmonary pressure can be performed with an oesophageal balloon, which allows the chest wall and lung elastance to be separated (rather than measuring the elastance of the respiratory system as a whole)
- This may be still be excessive (or inadequate) in patients at extremes of chest wall elastance
- The plateau pressure is a substitute for the transpulmonary pressure, which reflects the actual elastic distending pressure of the lung
- Volutrauma
- Appropriate PEEP
- Neuromuscular blockade
- No evidence of benefit for routine use
- Appropriate for:
- Ventilator dyssynchrony despite deep sedation
- Very poor lung compliance preventing lung protective ventilation
- Advantages
- ↓ PTHx rate
- Predicate of proning
- Disadvantages
- Requires deep sedation
- ↑ Haemodynamic instability
- iNO
Appropriate to trial for hypoxaemia refractory to traditional ventilation, for:- Short periods
- As a bridge to proning or ECMO
- VV ECMO
- Lung protective ventilation
- E
- Early mobilisation
- G
- Stress ulcer prophylaxis
- Feeding
- H
- DVT prophylaxis
One reasonable approach to PEEP-setting is identifying the level where DO2 (i.e., the product of arterial oxygen content and cardiac output) is maximal. Determining this point at the bedside is, of course, difficult.
Disposition:
Marginal and Ineffective Therapies
- High-Frequency Oscillatory Ventilation
↑ Mortality compared to lung protective ventilation strategies. - Recruitment manoeuvres
Recruitment improves oxygenation by ↑ number of ventilated alveoli. However, following a recruitment manoeuvre:- Over-inflation of previously normal lung occurs
May be harmful. - Oxygenation may improve
Predominantly early ARDS with a lower baseline PEEP. - Haemodynamic instability may occur
Due to large changes in VR.
- Over-inflation of previously normal lung occurs
Recruitment manoeuvres are covered in more detail under Recruitment.
Anaesthetic Considerations
Complications
Prognosis
- Death
Improving with better preventative care and ↓ VILI. Mortality varies on:- Severity:
- ~35% mild
- ~40% moderate
- ~45% severe
- Cause and comorbidities:
- ↓ In trauma
- ↑ In elderly, chronic or acute organ failures, and sepsis
- Severity:
- Chronic lung disease
Pulmonary function tests normalise in 6-12 months. - Persistent critical illness
↑ In survivors of ARDS compared to non-ARDS ICU patients.- Functional impairment persists in young survivors of severe disease
Key Studies
Lung Protective Ventilation:
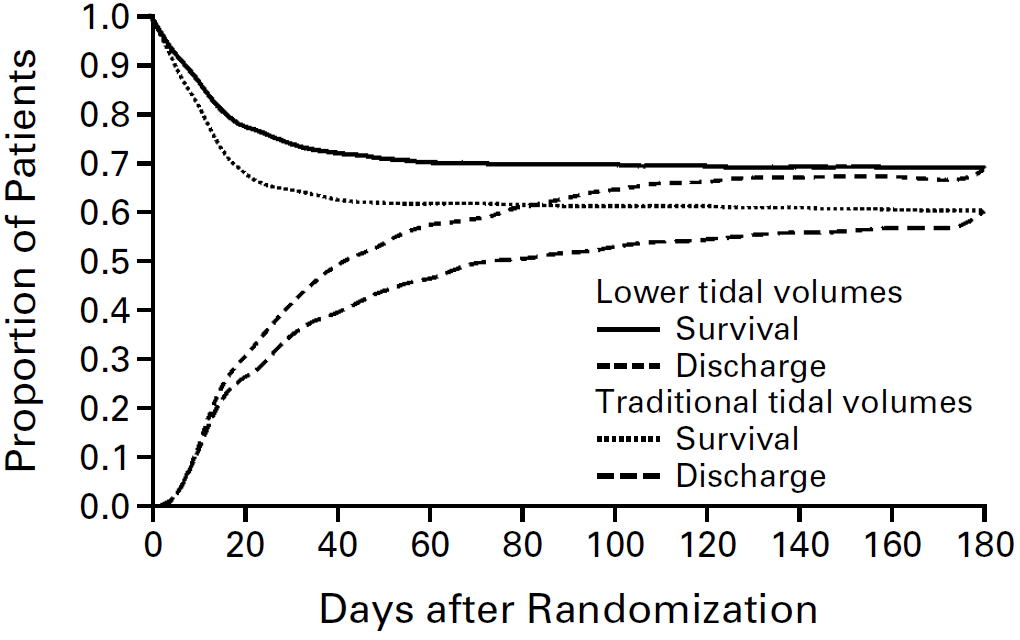
- ARMA (2000)
- 861 non-pregnant Americans with ARDS of any grade (P/F <300) without neuropathy, chronic lung disease, or severe burns
- Multicentre (10) randomised trial
- Low tidal volume ventilation vs. “traditional” high tidal volume ventilation
- Low tidal volume ventilation
- VCV at 6mL/kg VT
- Pplat <30cmH2O
- “Traditional” ventilation
- CVC with 12mL/kg VT
- Pplat <50cmH2O
- Low tidal volume ventilation
- Significant ↓ mortality (39.8% vs. 31%) in low tidal volume ventilation group
The ARMA trial also included a ketoconazole arm, which was stopped early for lack of efficacy. It has not been included in this summary.
| FiO2 | PEEP (cmH2O) |
|---|---|
| 0.3 0.4 0.4 0.5 0.5 0.6 0.7 0.7 0.7 0.8 0.9 0.9 0.9 1.0 1.0 |
5 5 8 8 10 10 10 12 14 14 14 16 18 18 20-24 |
Move up or down the table to target one of:
|
|
Open Lung Approach:
- Combination of high PEEP and recruitment manoeuvres to prevent alveolar collapse, and thereby:
- ↓ VILI
- ↓ Atelectotrauma
- ↑ Oxygenation
- (Appropriately) high PEEP is generally well established as safe and effective, the role of recruitment manoeuvres is unclear
- ART (2017)
- ~1000 adults with moderate-severe ARDS requiring mechanical ventilation, without high risk of barotrauma or escalating vasoactive requirements
- Multicentre (120!), unblinded, allocation concealed, block randomised trial
- Open lung vs. control
- Open lung
- PCV with driving pressure of 15cmH2O
- Muscle relaxation applied
- Staircase recruitment up to PEEP of 45cmH2O
- PEEP down-titrated in 3cmH2O increments, with measurement of static compliance at leach level
Level with highest lung compliance noted. - Further recruitment at PEEP 45cmH2O of PEEP
- New PEEP set to level at maximal compliance + 2cmH2O
- Control
- Conventional ARDSnet ventilation
- Both groups managed with VCV at 6mL/kg with safe lung ventilation
- Refractory hypoxaemia managed with proning, iNO, or VV ECMO
- Pressure support attempted once PEEP <14cmH2O
- Open lung
- ↑ Mortality in open lung group (55.3% vs 49.3%, p=0.04)
- Secondary outcomes showed the open lung group had ↑ 6 month mortality, ↓ ventilator free days, and more pneumothoraces requiring drainage
- Compliance in open lung group did not significantly ↑ following recruitment
- Recruitment manoeuvre had to be abandoned in 15% due to hypotension or hypoxia
- Most patients only had one recruitment manoeuvre
- Indiscriminate aggressive recruitment manoeuvres are harmful in moderate-severe ARDS, but this does not rule out benefit in select subgroups
Muscle Relaxation:
- Lung protective ventilation aims to ↓ VILI by ↓ barotrauma and Pip
- NMBA bypass any ventilator dyssynchrony and may improve chest wall compliance
- ACURASYS (2010)
- 340 French adults intubated with ARDS for <48 hours with a P/F ratio <150mmHg
- Multicentre (20), double-blind, block randomised trial
- 80% power to detect 15% ↓ ARR (!) in 90 day mortality assuming control mortality of 50%
- Cisatracurium vs. placebo for 2 days
- Cisatracurium
15mg bolus then 37.5mg/hr. - Placebo
- All patients received VCV and ARDSnet ventilation with 6-8mL/kg VT
- Cisatracurium
- Significant ↓ in adjusted 90 day mortality (Hazard Ratio 0.68, CI 0.48-0.98)
- Underpowered due to lower than expected control mortality
- Secondary outcomes:
- No difference in actual mortality (32% vs. 41%)
- ↓ Barotrauma in cisatracurium group
- ↑ Organ failure days in cisatracurium group
- ROSE (2019)
- 1006 Americans with moderate-severe ARDS (P/F <150), with several appropriate exclusions including BMI >100
- Multi-centre (48), unblinded, block randomised trial
- 1408 patients provide 90% power for 9% ↓ mortality, based on control group mortality of 35%
- Deep sedation and paralysis vs. light sedation for average 48 hours
- Intervention
- Deep sedation
- Cisatracurium
15mg bolus then 37.5mg/hr.
- Control
- Light sedation
- 25% received NMBA
- Sedation to RASS 0 to -1
- Managed with high PEEP
- Intervention
- Stopped early for futility
- No change in mortality (42.5% vs 42.8%)
- Secondary outcomes: ↑ Cardiac SAE in intervention group
- 80% of screened patients excluded, with a large number already receiving NMBA
Other:
- PROSEVA (2013)
- 474 Europeans with ARDS (PF <150mmHg), intubated for <36 hours at inclusion
- Multicentre (experienced proning units), assessor-blinded, RCT
- 456 patients gives 90% power for 15% ARR from control mortality of 60%
- Randomised to proning vs. supine
- Proning
16 consecutive hours for 28 days, or until improvement. - Standardised ventilation and weaning strategy
- Proning
- Significant ↓ mortality (16% vs. 33%, OR 0.42 (0.26-0.66), NNT 6) in proning group
- Over 2000 patients were not screened
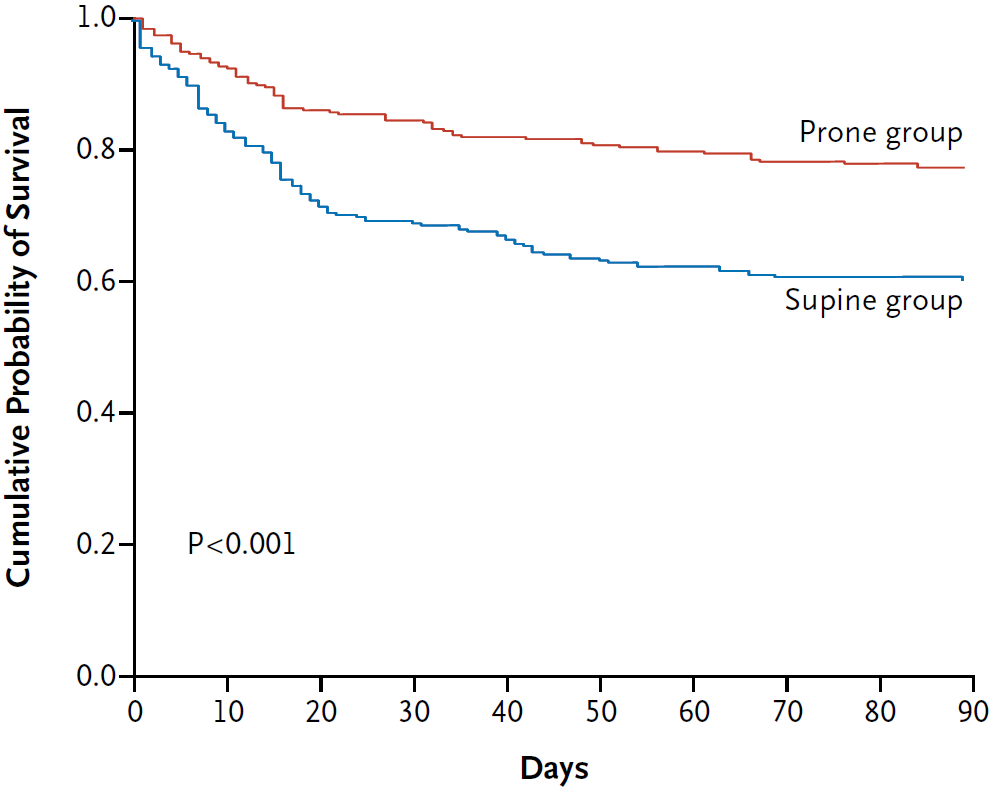
PROSEVA was the definitive trial on proning.
- ARMA (2000)
- 861 non-pregnant Americans with ARDS of any grade (P/F <300) without neuropathy, chronic lung disease, or severe burns
- Multicentre (10) randomised trial
- Low tidal volume ventilation vs. “traditional” high tidal volume ventilation
- Low tidal volume ventilation
- VCV at 6mL/kg VT
- Pplat <30cmH2O
- “Traditional” ventilation
- CVC with 12mL/kg VT
- Pplat <50cmH2O
- Low tidal volume ventilation
- Significant ↓ mortality (39.8% vs. 31%) in low tidal volume ventilation group
The ARMA trial also included a ketoconazole arm, which was stopped early for lack of efficacy. It has not been included in this summary.

| FiO2 | PEEP (cmH2O) |
|---|---|
| 0.3 0.4 0.4 0.5 0.5 0.6 0.7 0.7 0.7 0.8 0.9 0.9 0.9 1.0 1.0 |
5 5 8 8 10 10 10 12 14 14 14 16 18 18 20-24 |
Move up or down the table to target one of:
|
|
- DEXA-ARDS (2020)
- 277 non-pregnant, non-lactating adult Spaniards intubated for moderate-severe ARDS undergoing active treatment
- Without COPD, heart failure, or other immunosuppressants
- Assessor (not clinician) blinded, multicentre (17) randomised trial, stratified by centre
- 80% power to detect ↓ from 9 to 7 ventilator free days, and 15% ↓ (!) in 60 day mortality assuming control mortality of 48%
- Dexamethasone vs. usual care
- Dexamethasone group
- 20mg IV OD from day 1-5
- 10mg IV OD from day 6-10
- Dexamethasone group
- Significant ↑ in ventilator-free days (12.3 vs 7.5)
- Secondary outcomes of 60 day mortality, ICU mortality, duration of mechanical ventilation, P/F ratio all favoured dexamethasone group
- Most patients not proned
- Most patients had pneumonia or sepsis
- Control group mortality lower than anticipated
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. The Lancet Respiratory Medicine. 2020;8(3):267-276. doi:10.1016/S2213-2600(19)30417-5
- Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2019;380(21):1997-2008. doi:10.1056/NEJMoa1901686
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2017;318(14):1335-1345. doi:10.1001/jama.2017.14171
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2010;363(12):1107-1116. doi:10.1056/NEJMoa1005372
- Guérin C, Reignier J, Richard JC, et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2013;368(23):2159-2168. doi:10.1056/NEJMoa1214103
- Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone Infusion in Early Severe ARDS: Results of a Randomized Controlled Trial. Chest. 2007 Apr 1;131(4):954–63.