Aspergillosis
Fungal infection by the Aspergillus mould family with a broad spectrum of clinical disease from:
- Hypersensitivity (allergic bronchopulmonary aspergillosis)
Colonisation is usually benign, but allergic bronchopulmonary aspergillosis can result and progress to:- Asthma
- Bronchiectasis
- Pulmonary fibrosis
- Aspergilloma
- Necrotising pneumonia
- Invasive aspergillosis
Severe respiratory failure, usually with different preceding course depending on immune function:- Neutropenic
- Refractory fever
- Extra-pulmonary infection
Abscesses. - Pulmonary infarction
- Non-neutropenic
Mimics bacterial pneumonia with dyspnoea and sputum production as the dominant features.
- Neutropenic
Epidemiology and Risk Factors
Major risk factors for both disease and severity are essentially surrogates of immunosuppression as well as underlying lung disease. They include:
Respiratory disease significantly ↑ rate of aspergillus colonisation, and risk of subsequent infection if immunocompromise occurs.
- Patient
- Extremes of age
<1 and >70. - Malnutrition
- Alcohol misuse
- Extremes of age
- Disease
- Respiratory
- COPD
- CF
- Interstitial lung disease
- Immunocompromise
- Diabetes
- Burns
- HIV
- Prolonged neutropenia
- Bone marrow transplant
- Solid organ transplant
Lung most significant. - Complicated abdominal surgery
- Decompensated CLD
- Respiratory
- Treatment factors
- Prolonged ICU stay
- Mechanical ventilation
- Broad spectrum antibiotics
- Indwelling catheters
- PN
- Steroid
>1mg/kg for >2 weeks. - Chemotherapy
- Environmental
- Construction site exposure
Pathophysiology
Fungal pneumonia:
- Peri-vascular predominance in the immunocompromised
- ↑ Rate of pulmonary infarction
Leads to:- Haemoptysis
- Cavity formation
- ↑ Rate of pulmonary infarction
- Bronchoalveolar pneumonia in the immunocompetent
- Similar to other pneumonias
- “Classical” features of cavitation and aspergilloma formation are rarer
Clinical Features
Features include:
The majority of cases are secondary mycoses of the immunosuppressed, although can occur in otherwise immunocompetent patients with intercurrent illness.
- Respiratory failure
- Resting hypoxaemia
- Persistent infection
- Pneumonia refractory to antibiotics
- Refractory fever
- Pulmonary infarction
- Cough
- Pleuritic chest pain
- Haemoptysis
May be massive. - Pneumothorax
Rare.
- Distant infection
- Abscesses
- Skin lesions
In the immunocompromised, haematogenous dissemination and rampant infection can lead to:
- Endocarditis
- Endophthalmitis
- Abscesses
Investigations
Cultures, stain, and specific testing (galactomannan, PCR) are unable to discriminate between colonisation and infection.
Laboratory:
Galactomannan is a cell wall polysaccharide found in aspergillus, ice cream, salad dressings, and cream cheese.
- Blood
- FBE
- Eosinophilia
- Neutropenia
- Serum galactomannan
- Moderately sensitive (75%) and specific (85%) at a cutoff of >0.5
Higher cutoffs ↑ specificity and ↓ sensitivity. - Lower sensitivity in the immunocompetent and those receiving prophylaxis
- Moderately sensitive (75%) and specific (85%) at a cutoff of >0.5
- (1→3)-β-D-glucan antigen
Cell wall component of most fungi. Improves sensitivity and specificity in combination with galactomannan. - Serum PCR
Similar diagnostic properties to serum galactomannan.
- FBE
- Bronchoscopy
BAL is an integral part of adequate sampling.- Visual inspection
- Fungal culture and stain
- Difficult to culture, with correspondingly low sensitivity and long culture times
- Loss of specificity with aspergillus colonisation
- Aspergillus PCR
- Highly sensitive - negative result persuasive for no infection
- BAL galactomannan
- Better than serum galactomannan in the immunocompetent
More likely to have contained pulmonary disease.
- Better than serum galactomannan in the immunocompetent
- Transbronchial biopsy
- Definitive diagnostic test
- Risk of bleeding and pneumothorax often precludes this, particularly in the critically unwell
- Sputum
Significantly ↓ yield compared with BAL.- Aspergillus radioallergosorbent assay
- Silver staining
- Aspergillus IgG/IgE
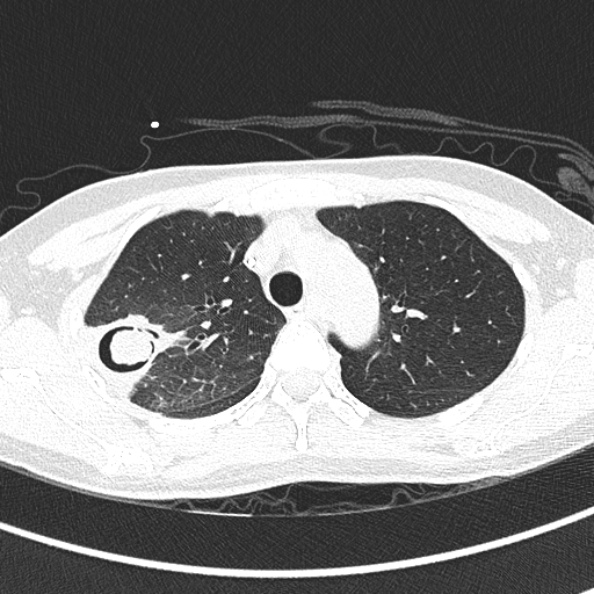
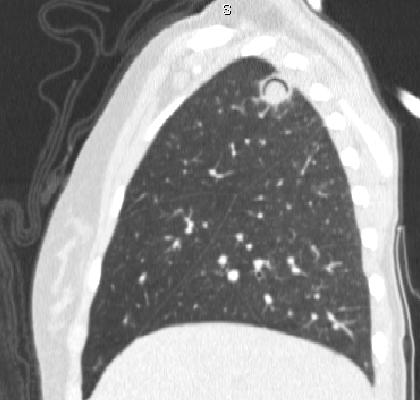
Imaging:
- CXR
- Aspergilloma cavities
- CT
- Aspergilloma cavities
- Nodules
- Monod sign
Free-moving (i.e. positional and dependent) aspergilloma in a pulmonary cavity. - Halo sign
Ground-glass opacity around a nodule or mass, usually indicating haemorrhage secondary to pulmonary infarction. - Air crescent sign
Halo of air around a region of necrosis; indicates recovery.
Other:
- Open lung biopsy
Rarely indicated due to risk of procedure.
Diagnostic Approach and DDx
Management
Aggressiveness of antifungal treatment depends on the severity of clinical presentation, and usually begins prior to diagnostic confirmation due to the long lead time of specific investigations.
Specific therapy:
- Pharmacological
- Allergic Bronchopulmonary Aspergillosis
- Corticosteroids
- PO itraconazole
- Aspergilloma
- PO itraconazole
- Pneumonia and invasive disease
- IV antifungal
- Voriconazole
1st line for efficacy. - Echinocandins
Synergistic when combined with azoles. Combination therapy may be appropriate with:- Known or likely azole resistance
From culture findings, treatment failure, or local microbial patterns. - Severe disease
Profound immunosuppression.
- Known or likely azole resistance
- Amphotericin B
Generally not preferred due to nephrotoxicity. Indicated for:- Hepatic failure
- Azole-resistant
- Voriconazole
- Altering immunosuppression
Consideration should be given to ↓ exogenous immunosuppression. - G-CSF
For neutropenia.
- IV antifungal
- Allergic Bronchopulmonary Aspergillosis
- Procedural
- Aspergilloma
- Surgical resection
- Percutaneous amphotericin injection
- Aspergilloma
- Physical
- Respiratory isolation
Supportive care:
Disposition:
Marginal and Ineffective Therapies
Anaesthetic Considerations
Complications
- Death
- B
- Pneumothorax
- I
- Disseminated infection
Prognosis
High mortality with invasive disease:
- 50-90%
Highest in the immunosuppressed.