Cardiac Arrest
This covers cardiac arrest. Components of post-arrest care, including interventions performed immediately following ROSC (e.g. angiography) are covered under Post-Resuscitation Care.
Epidemiology and Risk Factors
In-hospital Cardiac Arrest
- Significant variability in reported data
- ~1/1000 admissions
- Cardiac arrest team callout in 30/1000 admissions
- Majority are expected and occur without attempt at resuscitation
- ~80% of patients have deterioration in clinical signs during the few hours before cardiac arrest
Out-of-hospital Cardiac Arrest
- 50-150/100,000 person-years
Pathophysiology
Aetiology
Prodromal symptoms provide clues to the diagnosis:
- Headache
- SAH
- Chest pain
- Myocardial ischaemia
- PE
- Dyspnoea
- MI
- PE
- Other respiratory cause
Investigations
Management
- Call for help
- Commence BLS
- Determine appropriateness for ongoing resuscitation
If unclear, or if concerns exist.
- Determine appropriateness for ongoing resuscitation
- Early defibrillation and ALS
- Identify and correct reversible causes
- Commence post-resuscitation care
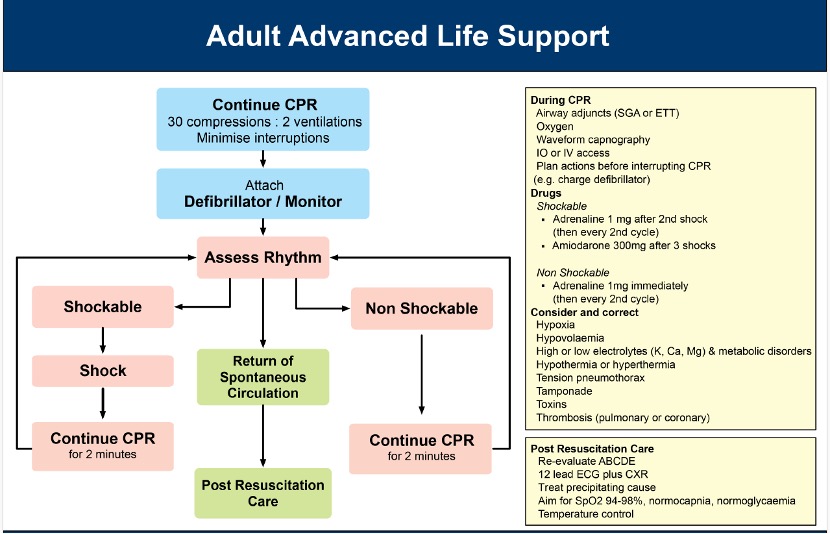
This management section focuses on the emergency management of cardiac arrest. Post-arrest care is covered under Post-Resuscitation Care.
The concept of the chain of survival recognises that successful resuscitation from cardiac arrest requires rapid and early completion of several constituent steps performed in series.
Appropriateness of Resuscitation
Considerations for determining appropriateness for resuscitation include:
- Resuscitation status
- Likelihood of good functional outcome
- Age
- Severe underlying disease
- Malignancy
- Severe cardiac failure
- Severe COPD
- Dementia
- Low-flow/no-flow duration
- Witnessed arrest
Unwitnessed strongly associated with poor outcome. - Bystander CPR
- Quality
- Time to initiation
>10 minutes strongly associated with poor outcome.
- Duration of bystander CPR
- Witnessed arrest
Basic Life Support
Pillars of basic life support:
Bystander CPR ↑ the change of survival by 2-4× after VF/VT arrest.
Signs of life include:
- Ventilator dyssynchrony
- Coughing
- Eye opening
- Swallowing
- Gasping
- Limb movement
- Swallowing
- Lacrimation
- Danger
- Response
- Send for help
- Open Airway
- Normal Breathing?
- Start CPR
- 30 compressions:2 breaths
- 100 compressions/minute
- 1/3rd depth of chest
- Lower half of sternum
Above this is less effective, below has ↑↑ risk of solid organ injury.
Advanced Life Support
In a witnessed and monitored arrest, with pads already attached, it is ideal to perform 3 stacked (repeated) shocks prior to commencing CPR.
In a witnessed and monitored arrest, without pads, it is reasonable to perform a precordial thump.
Defibrillation within 3-5 minutes of arrest can produce survival rates of 50-70%.
- A
- Airway
Adequate airway for gas exchange is the goal. Attempts at securing a definitive airway may excessively prolong time without CPR or gas exchange, without improving survival.
- Airway
- B
- End-tidal CO2
- Correlates with cardiac index
- Allows detection of ROSC
- End-tidal CO2
- C
- Attach Defibrillator
- 200J biphasic or 360J monophasic
- Anterior-Posterior pad position may be slightly preferable as it is less dependent on heart position
- Attach Defibrillator
Reversible Causes
Identify and correct reversible causes:
- Hypoxia
- Hypovolaemia
- Hyper/hypokalaemia
- Hypo/hyperthermia
- Tension pneumothorax
- Tamponade
- Toxins
- Thrombosis
- Coronary
- Pulmonary (embolism)
Marginal and Ineffective Therapies
Complications
- Death
- C
- Ischaemic injury
- CPR complications
- Rib/sternal fractures
- Solid organ injury
- D
- Hypoxic brain injury
- Seizures
In ~36% of patients after ROSC.
Prognosis
In-hospital Cardiac Arrest
Has a surprisingly good overall prognosis:
Neuroprognostication is covered under Neuroprognostication.
- Hospital discharge in 52%
Multifactorial:- Early detection
- Early arrival of ALS
Out-of-hospital Cardiac Arrest
Significantly poorer prognosis. Of those patients requiring ICU admission is significantly worse:
- ~60% die with HIE
The most common cause of death is withdrawal of life sustaining therapy. - Overall 12% survive to hospital discharge
- 30% survival in patients presenting with shockable rhythm
Key Studies
Many key trials target only OOHCA or IHCA patients, even though therapy could conceivably benefit either group.
It is unclear how generalisable results in one group are to the other, given the disparities in aetiology and outcome.
This includes key trials on the management of cardiac arrest. Trials evaluating interventions that occur after achieving ROSC (e.g. angiography, hypothermia) are covered under Post-Resuscitation Care.
ECMO:
- Observation studies indicate improved survival following OOHCA with ECMO compared to standard care
- ARREST (2020)
- 30 non-pregnant adult Minnesotans with shockable OOHCA without ROSC in ⩽3 shocks, within 30 minutes of study centre
- Without contraindications to resuscitation, or arrest from drowning, trauma, burns, or overdose
- Single-centre phase II open-label RCT
- Randomised to immediate cath lab with VA ECMO by cardiology, vs. ED treatment with angiography if ROSC occurred
- All patients admitted to cardiac ICU, received TTM, and neuro-prognosticated after 72 hours
- Significantly greater survival (43% vs 7%) in ECMO group
Other:
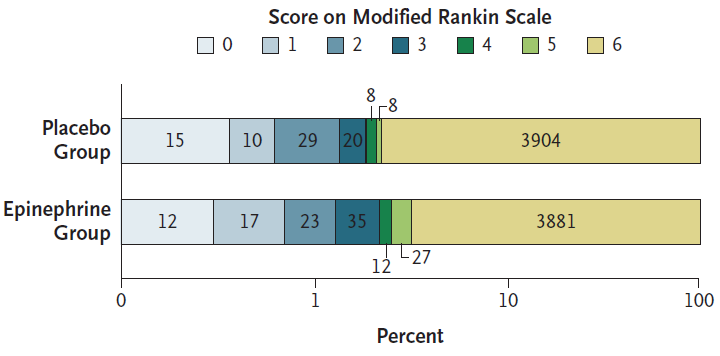
- PARAMEDIC 2 (2018)
- ~8000 Britons >16 with OOHCA undergoing ALS where adrenaline was indicated
- Multicentre (5 ambulance services), randomised, double-blind trial
- Powered for 1.5% ↑ in 30 day survival from a control group event rate of 6%
- Adrenalin vs. placebo
- Adrenaline group
- Average 4.9 doses
- 3980 shocks
- Placebo
- Average 5.1 doses
- 3962 shocks
- Adrenaline group
- 21% had initially shockable rhythm
- Adrenaline group had significantly ↑:
- ROSC (36% vs 11%)
- Transfer to hospital (50% vs 30%)
- Survival at 30 days (3.2% vs 2.4%)
- No difference in survival with favourable neurological outcome (mRS ⩽3)
- The survivors in the adrenaline group had ↑ severity of neurological impairment
- Reinforces the importance of good, early bystander CPR and defibrillation
- DOSEVF (2022)
- 405 Canadians patients with OOHCA arrest with refractory VF arrest
Refractory >3 consecutive rhythm checks demonstrating VF. - Initial shocks performed with A-L pad placement
- Groups randomised then to one of three groups:
- No change
- Vector change: Pads moved to A-P defibrillation
- Double-sequence: A-P pads added and sequenced (<1s between shocks) A-L and A-P defibrillation
- Double-sequence defibrillation resulted in significant ↑:
- ROSC (46% vs 26%)
- Survival to hospital discharge (30% vs 13%)
- Survival with mRS ⩽2 (27% vs 11%)
- Vector-change defibrillation resulted in significant ↑ survival to hospital discharge (21.7% vs 13.3%) but no significant change in ROSC or neurological outcome.
- Stopped early with a low number of outcomes; ↑↑ risk bias
Stoppage due to paramedic staffing shortages. - Median time to 1st shock ~10 minutes
- 405 Canadians patients with OOHCA arrest with refractory VF arrest
- COCA (2021)
- 397 adult non-pregnant Danes with non-traumatic OOHCA with >1 dose adrenaline
- Block randomised by ambulance station
- 5mmol calcium chloride (IV, IO) vs. saline placebo
- No difference in primary outcome of ROSC (19% vs 27%)
- No difference in 30 day survival, 90 day survival, or 30 day survival with good neurological outcome
- Significant difference in 90 day survival with good neurologic outcome (3.6% vs. 9.1%, RR 0.4 (0.17-0.91))
I feel this is probably dubious given the otherwise consistent findings and the wide confidence intervals.
- VAM-IHCA (2021)
- 392 adult Danes with IHCA requiring adrenaline, without invasive circulatory support, in 10 hospitals
- Investigator-initiated, blinded, placebo-controlled, block-randomised by site
- Consent from patients or surrogates, if survived
- Randomised to 40mg methylprednisolone and 20 units vasopressin vs. placebo
- Intervention group:
- Received cocktail as soon as possible after first adrenaline dose
- Further 20 unit vasopressin boluses could be given after each adrenaline dose
- Placebo group:
- Received otherwise usual care
- Notably significantly higher use of VA ECMO (30% vs 14%)
- Intervention group:
- Intervention group demonstrated significant ↑ in ROSC (42% vs 33%, CI 1.03-1.63)
- No difference in secondary outcomes of 30/90 day survival or neurological outcome
- No difference in TTM, angiography, or glucocorticoid use
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Schmidt H, Kjaergaard J, Hassager C, et al. Oxygen Targets in Comatose Survivors of Cardiac Arrest. New England Journal of Medicine. 2022;387(16):1467-1476. doi:10.1056/NEJMoa2208686
- Andersen LW, Isbye D, Kjærgaard J, et al. Effect of Vasopressin and Methylprednisolone vs Placebo on Return of Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326(16):1586–1594. doi:10.1001/jama.2021.16628
- Cheskes S, Verbeek PR, Drennan IR, et al. Defibrillation Strategies for Refractory Ventricular Fibrillation. N Engl J Med. 2022;387(21):1947-1956.
- Vallentin MF, Granfeldt A, Meilandt C, et al. Effect of Intravenous or Intraosseous Calcium vs Saline on Return of Spontaneous Circulation in Adults With Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326(22):2268–2276.
- Desch S, Freund A, Akin I, et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. New England Journal of Medicine. 2021;385(27):2544-2553.
- Lemkes JS, Janssens GN, Van Der Hoeven NW, et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N Engl J Med. 2019;380(15):1397-1407.
- Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. New England Journal of Medicine. 2021;384(24):2283-2294. doi:10.1056/NEJMoa2100591
- Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. The Lancet. 2020;396(10265):1807-1816. doi:10.1016/S0140-6736(20)32338-2
- Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. New England Journal of Medicine. 2019;381(24):2327-2337. doi:10.1056/NEJMoa1906661
- Perkins GD, Ji C, Deakin CD, et al.A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. New England Journal of Medicine. 2018;379(8):711-721. doi:10.1056/NEJMoa1806842
- Advanced Life Support Level 2 Third Australian Edition. Australian Resuscitation Council. 2016.