Vasopressors
Vasopressors are drugs that predominantly constrict peripheral vasculature, causing a:
Although we like to conceptually separate Inotropes from vasopressors, there is substantial overlap in the pharmacodynamic activity of many drugs in either category. Unlike inotropes however, there are some pure vasopressors.
My preference for nomenclature is to use the umbrella term vasoactive for all agents used to manipulate haemodynamic parameters, and then categorise them based on their primary function:
- Inotrope for agents that ↑ the force of myocardial contraction
- The subcategory inodilator for drugs which also ↓ SVR
- Vasopressor for agents that ↑ SVR
- Vasodilator for agents that ↓ SVR
- Primary ↑ in SVR, causing a secondary ↑ in MAP
- Primary ↑ venous tone which ↓ venous compliance and ↑ stressed volume, causing a secondary ↑ in preload
Venous blood volume can be divided conceptually into:
- Unstressed volume
Does not exert meaningful pressure on the walls of the vasculature, and so does not contribute to MSFP. Contributes ~85% of total vascular volume under normal circumstances. - Stressed volume
Exerts pressure on the walls of the vasculature and therefore produces MSFP.
Indications
Vasopressors are used:
- To correct hypotension-induced hypoperfusion
i.e. When there is:- Evidence of ↓ end-organ perfusion
- ↓ MAP
- Despite adequate volume state
- Despite adequate pump function
- For therapeutic hypertension
- TBI
- DCI
- Spinal cord ischaemia
Blood Pressure Targets
This applies to targets in the hypotensive, hypoperfused patient.
Physiological rationales exist for a variety of targets:
- High MAP targets
- ↓ AKI by improving renal perfusion pressure
- May ↑ cerebral perfusion and improve neurological outcomes
- Provides margin of safety for hypotensive episodes
- Lower MAP associated with ↑ mortality
- Lower MAP targets
- ↓ Dose of vasopressors required
- ↓ Arrhythmia
- ↓ Myocardial work
- ↓ ICU length of stay
- ↓ Fluids, steroids, etc. administered
- Escalating doses may not be met with ↑ pressure, resulting in very high doses used
- ↓ Dose of vasopressors required
In general:
- MAP 65-70mmHg is reasonable in most patients in the resuscitation phase
- Lowering (to MAP 60mmHg) in the elderly to ↓ vasoactive requirements and harm of administration
- If there is evidence of end-organ perfusion, trial of a vasopressor-driven MAP challenge is reasonable
Temporarily ↑ MAP target to evaluate for a given physiological response (e.g. ↑ UO.
Contraindications
Comparison
| Drug | Overview | Advantages | Disadvantages |
|---|---|---|---|
| Noradrenaline | |||
| Vasopressin | Endogenous nonapeptide Acts on V1 and V2 receptors |
|
|
| Methylene Blue |
|
|
Equipment
Technique
Complications
Key Studies
Targets:
- ANDROMEDA-SHOCK (2019)
- 424 adult south Americans with septic shock requiring vasopressors despite 20mL/kg volume resuscitation
- Multicentre (28), randomised, clinician unblinded, assessor blinded
- 90% power for 15% ARR (!) for mortality
- Peripheral perfusion-targeted resuscitation vs. lactate targeted resuscitation
- Peripheral perfusion
- Finger pad capillary refill time Q30 minutely, then hourly until normalisation
- Targeted capillary refill time <3s
- Lactate targeted
- Lactate measured Q2H for 8 hours
- Targeting ↓ lactate by 20% every 2 hours until normal
- Failure to normalise was targeted with escalating protocols:
- Fluid responsiveness
- PPV or VTI change following passive leg raise
- 500mL crystalloid
- Vasopressor test
- MAP ↑ to 80-85
- If target normalised, MAP maintained
- Otherwise MAP target set to 65
- MAP ↑ to 80-85
- Inodilator test
- Dobutamine or milrinone
- Fluid responsiveness
- Peripheral perfusion
- ↑ Mortality in lactate group (43.4% vs 34.9%)
- Underpowered for outcome
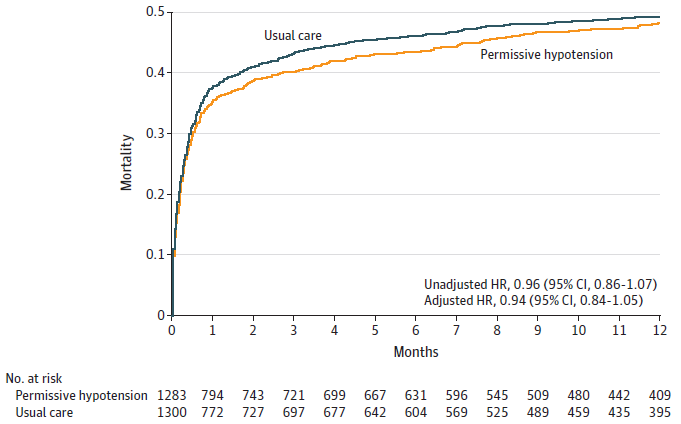
- 65 (2020)
- 2600 Britons >65 years old with vasopressors for hypotension secondary to ↓ SVR
- Following fluid resuscitation and without contraindications to permissive hypotension
- Multi-centre (65), parallel group, unblinded RCT
- 90% power to detect 6% ARR in mortality relating to permissive hypotension
- Randomised to permissive hypotension vs. standard care
- Permissive hypotension
- MAP targeted 60-65mmHg
- Standard care
MAP targets at clinician discretion. - Vasopressors used at clinician discretion
- Permissive hypotension
- No difference in 90 day mortality (41% vs 43.8%)
- Subgroup with chronic hypertension had lower mortality with permissive hypotension (38% vs. 55%, OR 0.67, CI 0.49-0.85)
Not powered for this.
- Subgroup with chronic hypertension had lower mortality with permissive hypotension (38% vs. 55%, OR 0.67, CI 0.49-0.85)
- No difference in SAE
- No difference in secondary outcomes of ICU discharge, duration of ventilation, CRRT, or cognitive outcome
- Indicates lowering MAP targets to ↓ vasopressor exposure is safe in vasodilatory hypotension in the elderly
Vasopressin:
- VAAST (2008)
- ~800 adults with septic shock, without mesenteric ischaemia, hyponatraemia, or TBI
- Double-blind, randomised trial, stratified by shock severity
- 80% power to detect 10% (!) ↓ ARR from baseline mortality of 60%
- Vasopressin vs. noradrenaline
- Vasopressin up to 0.03 units/minute (3.6 units/hr)
- Noradrenaline up to 15μg/minute
- Open label infusion if maximal rate reached
- Cross-over to vasopressin was not allowed for noradrenaline group
- No change in 28 day mortality (35.4% vs 39.3%)
- Vasopressin is safe, at least up to ~4 units/hr
The VAAST protocol provides a crude dose-conversion between noradrenaline and vasopressin, with 1 unit/hr of vasopressin being roughly equivalent to 8μg/minute of noradrenaline.
- VANISH (2016)
- 421 adults with sepsis requiring vasopressors despite adequate fluid resuscitation, without steroids, mesenteric ischaemia, or vasospastic disease
- 80% power for 25% ↓ in RRT with an control group incidence of ~30%
- Vasopressin + hydrocortisone or placebo vs. noradrenaline + hydrocortisone or placebo
- Vasopressin
- Vasopressin titrated up to 3.6 units/hr
- At maximal rate, hydrocortisone 50mg IV Q6H or placebo commenced
Given for 6 days, then weaned over 6 days.
- Noradrenaline
- Noradrenaline titrated up to 12μg/min
- At maximal rate, hydrocortisone given as above
- If hypotensive after first dose of hydrocortisone (or placebo), open-label catecholamines could be commenced
These were weaned as a priority.
- Vasopressin
- No difference in days alive free of AKI
- ↓ RRT in vasopressin group
Other:
- Bellomo et al (2000)
- 230 adults with ⩾SIRS features, a central line, and a sign of early renal dysfunction; without significant CKD, renal transplant, or recent AKI
- Multicentre (23), block randomised, double-blind, placebo-control trial
- 80% power for 20% ↓ in serum creatinine
- Dopamine vs. placebo
- Dopamine at 22µg/kg/min
- Placebo infusion at same rate
- Continued until death, RRT, ICU discharge, or resolution of SIRS and renal dysfunction
- No change in serum creatinine
- No change in secondary outcomes, notably arrhythmia
- No change in urine output in dopamine group
- MIDAS (2020)
- 134 non-pregnant adult American and Australian ICU patients requiring a single vasopressor at a low dose for >24 hours following resuscitation and treatment of reversible causes, without evidence of hypovolaemia, cardiac failure, CKD
- Multi-centre (3), double-blind, placebo-controlled, randomised, stratified by site
- 100 patients required to detect 6 hour difference in time to vasopressor cessation
- Midodrine 20mg Q8H vs. placebo
- No difference in time to discontinuation of vasopressors (23.5 hours vs 22.5 hours) or ICU discharge
- ↑ Bradycardia (HR <40) in midodrine group (8% vs 0%)
- CENSER (2018)
- 456 adults with hypotension and sepsis, without septic shock or other significant acute disease
- Single-centre, blinded RCT
- 300 patients provides 80% power to detect 20% ↑ “shock resolution” at 6 hours, compared to 60% in control group
MAP sustained >65mmHg >15 minutes with >2 hours of UO >0.5mL/kg/hr or ↓ lactate by >10% from initial level. - Noradrenaline vs. placebo
- Noradrenaline
- 0.05μg/kg/min for 24 hours without titration
- D5W placebo
- Open-label vasopressors if MAP <65
- Noradrenaline
- Greater shock resolution at 6 hours in noradrenaline group (76% vs 48%)
- More interestingly, over half of the patients had norad given peripherally and no extravasation injuries occurred
- ATHOS-3 (2017)
- 344 adults with refractory vasoplegic shock (>0.2μg/kg/min catecholamines for 6-48 hours with evidence of ↑ CI, without severe burns, ACS, mesenteric ischaemia, active haemorrhage, or mechanical support
- Double-blind, allocation concealed, block randomised, multicentre (75), international RCT
- 90% power to detect response in MAP (>75mmHg or >10mmHg ↑) at 3 hours, without ↑ in background vasopressors, assuming 40% response in placebo group
- Angiotensin II vs. placebo
- ATII
- 20ng/kg/min, titrated to MAP >75mmHg or 200ng/kg/min over 3 hours
- After 3 hours, titrated to 1.25-40ng/kg/min
- Other vasopressor dose held constant, safety permitting
- Adjustment of background vasopressors indicated “non-response”
- Placebo
- Placebo titrated by same profile
- ATII
- Significant ↑ in MAP in the ATII group (69% vs. 23%), with ↓ dose of background vasopressors
- No changes in patient relevant secondary endpoints
- Demonstrates efficacy and somewhat demonstrates safety
References
- Bersten, A. D., & Handy, J. M. (2018). Oh’s Intensive Care Manual. Elsevier Gezondheidszorg.
- Santer P, Anstey MH, Patrocínio MD, et al. Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): an international randomised clinical trial. Intensive Care Med. 2020;46(10):1884-1893. doi:10.1007/s00134-020-06216-x
- Lamontagne F, Richards-Belle A, Thomas K, et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA. 2020;323(10):938-949. doi:10.1001/jama.2020.0930
- Gordon AC, Mason AJ, Thirunavukkarasu N, et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016;316(5):509-518. doi:10.1001/jama.2016.10485
- Russell JA, Walley KR, Singer J, et al. Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock. New England Journal of Medicine. 2008;358(9):877-887. doi:10.1056/NEJMoa067373
- Khanna A, English SW, Wang XS, et al. Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 2017;377(5):419-430. doi:10.1056/NEJMoa1704154
- Bellomo R, Chapman M, Finfer S et al. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. The Lancet. 2000;356(9248):2139-2143. doi:10.1016/S0140-6736(00)03495-4